An eQMS is a digitalized Quality Management System where you can create, store, retrieve, and archive all of your company’s quality-related documents. In the context of a life sciences company, an eQMS also automates compliance requirements under 21 CFR Part 11, and EU Annex 11 by allowing user-specific login credentials for security, electronic signatures, access levels for data protection, and maintaining audit trail reports for data integrity. An ideal eQMS for a life sciences company should have features to align with ISO 13485. If your company is beginning its digital transformation journey, then an eQMS is the right choice for you.
Digitalization addresses visibility issues in the quality management of life sciences companies. Therefore, many life sciences companies are switching from traditional paper-based QMS to eQMS. Knowingly or unknowingly these companies are participating in the Industry 4.0 revolution by choosing an eQMS over a paper-based system.
Why switch from a paper-based system to an eQMS?
There are several reasons why companies are making massive moves to switch from traditional paper-based systems to eQMS. Some of these reasons include :
Unorganized and Scattered Information
Paper-based systems result in unorganized documentation practices. Documents are scattered across employee cabinets and company locations. The older the system, the larger the number of quality management documents, and the more difficult it is to compile interrelated documents. Harmonizing document management practices and tying together all the pieces of information scattered across the company is the biggest challenge that companies face.
Untidy Practices
The use of Post-it notes, margins, footers, and headers to add new or missing information results in untidy documentation practices. This approach also raises questions about whether the document was signed before or after the addition of new information in vacant sections.
In addition to these untidy practices, poor handwriting, overwriting, and multiple strikethroughs in documents also impact document legibility in paper-based systems.
Companies need to establish internal protocols to minimize these practices. Examples of such practices include:
- Making it mandatory for employees to use indelible ink.
- Requiring a signature and date on every time new or missing information is added.
- Asking employees to strike through every empty page/empty portion of the page to avoid writing on the document after the document is signed off.
- Defining how employees should strike through incorrectly written letters or words. For example, don’t strike through a word more than once.
- Prohibiting overwriting.
Although the instructions mentioned above can minimize untidy practices in a company, they can never be zeroed down. At the end of the day, we are all human and bound to make a mistake sooner or later. To address these challenges effectively, the optimal solution is to delegate them to a computerized quality management system, minimizing incidents to zero.
Incomplete Documents
Another pressing issue that companies face is incomplete documents that were passed as complete documents by mistake. You may find incomplete root cause analysis, CAPAs, and even signatures in these documents. In a paper-based system, there is no way to notify workers of their pending actions, and there is no dashboard to glance at incomplete and unsigned documents, resulting in an ever-increasing pile of documents left unattended by its users.
Missing Documents
Missing QMS documents is another painful challenge that many companies face. As the organization grows, keeping track of physical document copies becomes more and more challenging. Employees might forget to turn in the master copies in their possession when they leave their job or are done with their duties. Some may not even know how many documents are scattered in their drawers. The real trouble comes when an auditor requests one of these missing documents. Even if you have a document management system, figuring out who is the possessor can take up a lot of your valuable time.
Considering all these challenges, transitioning from paper-based systems to an eQMS makes logical sense. Are you planning to switch from a paper-based QMS to an eQMS too? If so, continue reading to discover how you can overcome the challenges involved in the process.
Starting your transition
Planning your QMS transition process carefully is important. Below are some key steps, tips, and suggestions to make this transition hassle-free:
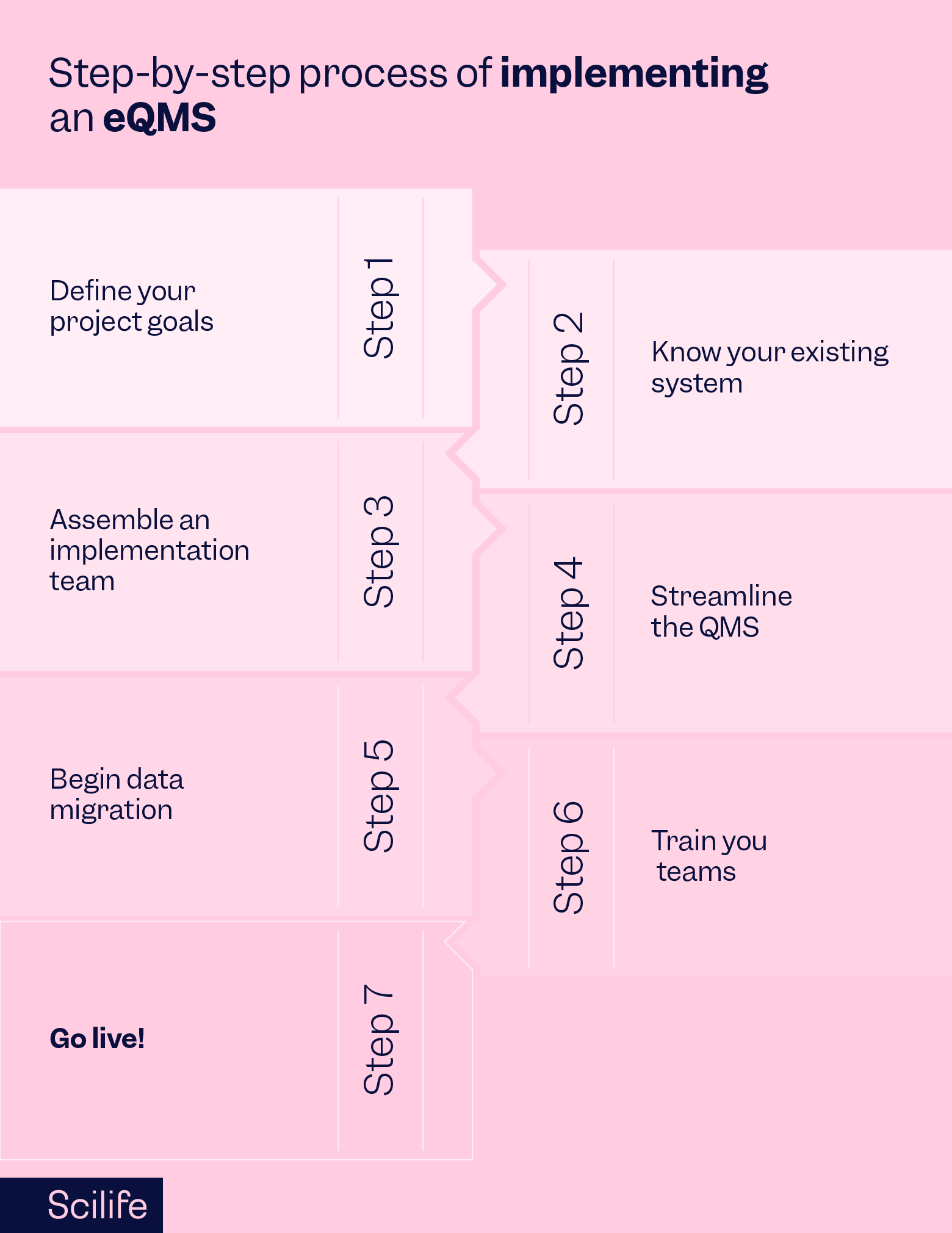
Define your project goals
Defining your targets in advance allows your employees to align their goals with your company’s QMS goals. Your targets may include specifics such as the go-live date, budget allocation, and anticipated improvements after the eQMS implementation.
Know your existing system
We all know the challenges in our existing systems, but knowing the system here means understanding the complete structure behind your existing QMS. It could begin with understanding your organization's structure such as identifying the number of departments (business functions) in your company. .
Assemble an implementation team
Changing your entire QMS is not one man’s job. You will need to create a team of champions with at least one champion coming from one business function/ department. The role of each champion will be to collaborate with the remaining employees in each department/ business function to facilitate a smooth transition.
Streamline the QMS
The first objective of the champion would be to work with their team and identify duplicate and redundant documents to streamline their processes. Once the processes are streamlined it will be a lot easier for you to begin the data migration to your new eQMS as it will help avoid duplication of the work in the next step.
At this stage, you can also consider working out the total number of employees who will need access to the eQMS and define their access levels based on their roles and responsibilities in the organization.
Begin data migration
A streamlined QMS structure will give you confidence to begin the data migration process. It is a lot easier for you to complete the data migration if your eQMS partner is Scilife. All you need to do is populate a spreadsheet with your documents' metadata and share their corresponding files. Our Customer Success team will guide you through this process.
Train your teams
The train-the-trainer concept works well with big organizations. Therefore, in big organizations, you will need to train your champions first. The champions will later train their respective teams. In smaller organizations, everyone can be trained at the same time.
Go live
When everyone is trained, you can go live. In cloud-based eQMS such as Scilife, this won’t be a time-consuming step as users don’t need to install anything on their end. You can quickly create the required users and their respective access levels. Additionally, we will collaborate with your IT team to work on features like Single sign-on. Therefore, if you are working with us, be assured that your transition from a paper-based system to an eQMS will be super smooth.
Conclusion
Switching from a traditional paper-based quality management system to an eQMS can provide numerous benefits for life sciences companies. Some of the key advantages include improved organization and accessibility, enhanced efficiency, robust compliance, and cost savings. However, the transition process itself requires thoughtful planning and execution.
By assembling an implementation team, streamlining existing QMS processes, methodically migrating data, and properly training all users, companies can ensure a smooth transition to their new eQMS. With the right strategic preparation and change management, organizations can avoid pitfalls and accelerate their digital transformation. The train-the-trainer approach and collaboration with expert eQMS partners like us further facilitates the changeover for employees across the business.
When the transition is carefully orchestrated, life sciences companies can seamlessly realize the many advantages that eQMS offers while minimizing disruption during the switch. Employees can adapt to the new paperless system and optimized workflows. This positions organizations for scalable growth and success. The benefits of eQMS make it a worthwhile endeavor for regulated businesses ready to digitally progress into the future, especially when shifts are gradually introduced and adoption is encouraged through supportive planning.
Discover how Scilife smart QMS can help you in your transition to an eQMS!