21 CFR Part 11 Compliance
What is 21 CFR Part 11?
The 21 CFR Part 11 is Part 11 of Title 21 of the Code of Federal Regulations. This regulation defines Electronic Records and Electronic Signatures requirements used by pharmaceutical or medical device manufacturers when they submit to the US FDA to show compliance with cGMP practices as mentioned in 21 CFR Part 211 or any other applicable regulatory requirements.
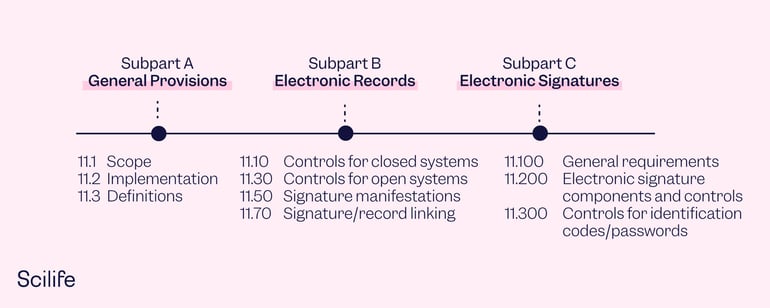
The exact location of the 21 CFR Part 11 is: Title 21 - Chapter I - Subchapter A - Part 11. Part 11 and Part 211 sound very similar but are entirely different sections of the 21 CFR. Part 211 is related to cGMP for Finished Pharmaceuticals, while Part 11 addresses Electronic Records and Electronic Signatures. Here, we’ll take a closer look at the Part 11 subparts.
The FDA issued its final Part 11 regulation in March 1997, which became effective on August 20, 1997. Ever since, the regulation has been revised from time to time to encourage electronic submission of records and minimize the cost of compliance. According to the 21 CFR Part 11, the definitions of electronic record and electronic signature are as follows:

Part 11 provides criteria for electronic signatures, records, and handwritten signatures, which are captured for an electronic record and are considered “equivalent to paper records and handwritten signatures executed on paper.” As signatures are used digitally on an electronic record, they must meet specific, regulation-outlined criteria if they fulfill Part 11 requirements.
PART 11 - Electronic Records; Electronic Signatures
A signature should contain three crucial pieces of information associated with the signing:
- The signer’s full name,
- The date and time when the signer signed, and
- The purpose of the signature such as review, approval, responsibility, or authorship.
Even if you’ve transformed your signature process into a digital platform, these three key pieces of information are still equally important and need to be recorded.
The primary reason for 21 CFR Part 11 compliance requirement is security and protection concerns about managing the distribution, storage, and retrieval of records by biotechnology, drug, and medical equipment manufacturers in the digital age.
Originally, the considerable cost of maintaining paper-based filing systems for these companies was intended to satisfy the regulator. A key objective of the new regulation was to enable these firms to adopt paperless systems.
The Guidance for Industry Part 11, Electronic Records; Electronic Signatures — Scope and Application guides manufacturers to meet 21 CFR Part 11 compliance by following these steps:
- Validation
- Audit Trail
- Legacy Systems
- Copies of Records
- Record Retention
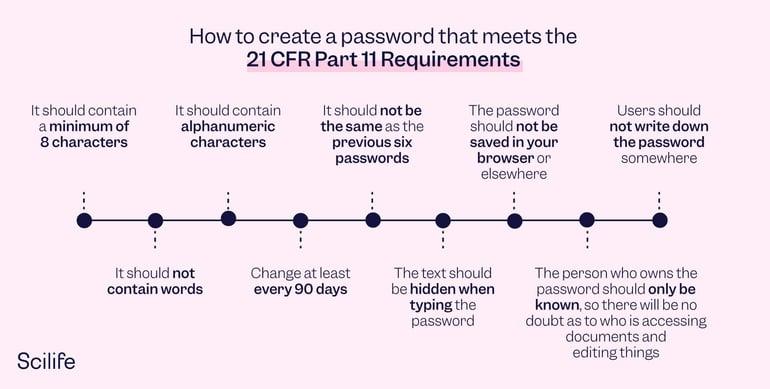
How can I create a password that meets the 21 CFR Part 11 requirements?
Does the software need validation?
Many companies use Good Automated Manufacturing Practices (GAMP) while some manufacturers prefer to focus on Part 11 requirements. To determine if a specific software in use requires validation, answer the following questions to find out:
What activities does this software cover?
Answer if the software creates, modifies, maintains, archives, retrieves, or transmits records under any requirement outlined in the regulations.
Will this software use electronic signatures?
Answer if the software requests a password from users.
What activities of this software cover?
After the software review, staff should generate documentation to ensure Part 11 compliance if the decision is made to validate. This documentation should contain the requirements for the intended use of the software.
Are all systems prevalidated?
This depends on the vendor of the system. For example, a system may already be designed to meet 21 CFR Part 11 requirements. In that case, the vendor can give you a documentation/pre-validated validation package so that you can demonstrate how the system complies and significantly decreases the workload of validation activities.
Any Life Sciences organization must fulfill the requirements of 21 CFR Part 11 or other related regulations to maintain records or submit designated information electronically. Therefore, if you’re planning to transform your paper-based Quality Management System (or QMS) into an electronic QMS, it is essential to know whether it is capable of 21 CFR Part 11 compliance.
To learn more about 21 CFR Part 11, we recommend reading up on Choosing a 21 CFR Part 11 Compliant QMS to answer the following key questions:
- Who should care about 21 CFR Part 11 compliance?
- How can a manufacturer meet 21 CFR Part 11 compliance?
- Is 21 CFR Part 11 applicable for manufacturers who are selling products in Europe?
Additional resources

How to Implement the Continuous Improvement Cycle | Scilife
Even an organization with stellar leadership and a solid core of employees experiences hiccups from time to time. Despite having assembled all the ...

How to assess and enhance your Quality Management Maturity | Scilife
As the life sciences industry becomes increasingly regulated and competitive, quality management has become more vital than ever. Are you confident ...

Best Quality Management Software (QMS) for Life Sciences | Scilife
The right electronic Quality Management System (eQMS) can help strengthen your compliance processes and build a culture of quality within your ...

How to write a good quality plan for medical devices | Scilife
In life sciences, especially if you’re in the medical device industry it becomes harder to manage projects in accordance with your company’s quality ...
Turn quality into your brightest asset with Scilife
