1
Automate all your processes. Gain a comprehensive quality management system.
1
Automate all your processes. Gain a comprehensive quality management system.
2
Bring all your teams together and empower them with a quality-first mindset.
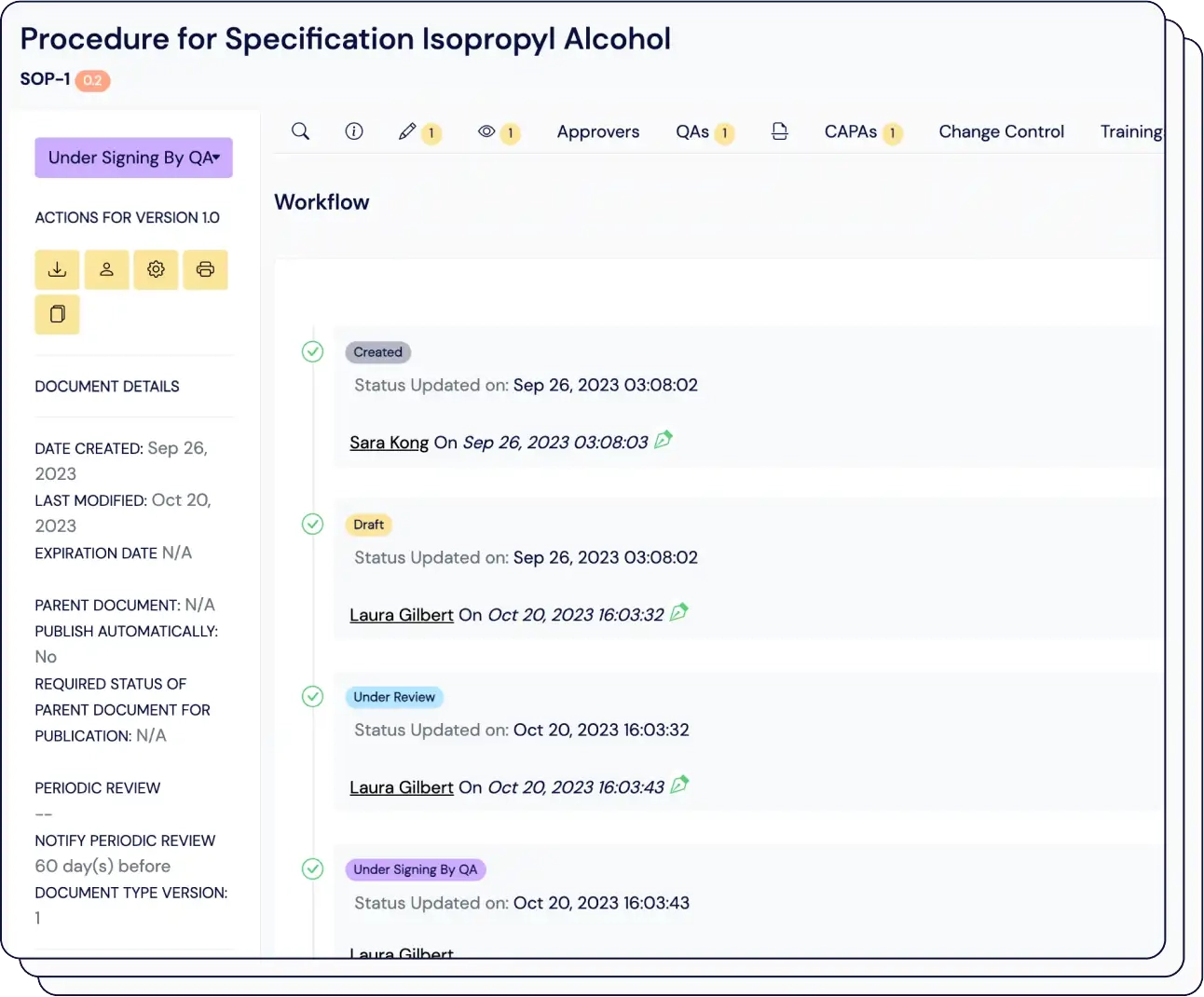
3
Achieve end-to-end traceability and data integrity compliance seamlessly within your workflow.
Digitalize your Document and Record Management and automate your Compliance Training with ease.
Ensure audit readiness, manage CAPAs, Deviations, and Nonconformities seamlessly. Integrate Change Control, and Risk Assessments for in-depth quality oversight.
Hit your KPIs and enhance performance with quality metrics. Foster collaboration and shared ownership of quality initiatives.
Eliminate confusion over tracking of elements in the design and development workflow, accelerating time-to-market by up to 35%.
Easily connect thousands of items, maintain version control, and automatically link risk assessments with just one click.
Compliant with ISO 13485, our traceability matrix shows how design inputs connect to user needs, and assess the impact of design changes on your validation and verification processes.

Integrate your essential apps for easy tracking and flawless audits, ensuring FDA 21 CFR 11 compliance.
Stay in the loop of who printed what, when and why. Recognize reprints automatically, so you can trace back every print action.
Use unique barcodes for full traceability to speed up and digitize the reconciliation and take action quickly to find missing documents.

At Scilife, your data security is our top priority. Understanding the critical importance of data security and compliance in the Life Sciences industry, we employ stringent measures across product and infrastructure security, monitoring, reliability, compliance, and privacy to keep your data safe.

Life sciences companies transform their teams into quality boosters with Scilife.
But don't just take our word for it!





EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
PRODUCT
INDUSTRIES
RESOURCES
COMPANY
Contact Us
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2025 Scilife N.V. All rights reserved.