While sometimes deprioritized, regulatory compliance is critical for medical device manufacturers. And among the global regulatory frameworks, the Medical Device Single Audit Program (MDSAP) has gained prominence over the past decade. MDSAP is a comprehensive approach to auditing and monitoring medical device manufacturers. It counts among its members some of the most significant regulatory markets in the world, such as the US, Brazil, Canada, Japan, and Australia.

This article reviews the intricacies of MDSAP, exploring its purpose, audit process, the entities involved, and, most importantly, how to effectively prepare for MDSAP audits.
If you’d like to see how MDSAP fits into the bigger picture of medical device quality systems—and explore other key regulations and standards—our QARA guide to medical device quality management systems is a must-read.
What is the Medical Device Single Audit Program (MDSAP), and what is its purpose?
MDSAP is an international initiative designed to streamline and harmonize the auditing process for medical device manufacturers. Established in 2012, MDSAP aims to enhance regulatory efficiency by allowing participating countries to leverage a single audit to satisfy the requirements of multiple regulatory authorities.
The program brings together its regulatory bodies to form a collaborative approach that will ensure the safety and effectiveness of medical devices. MDSAP seeks to eliminate redundancies, reduce regulatory burden, and enhance the overall quality of audits, ultimately benefiting both manufacturers and regulatory authorities.
Who conducts MDSAP Audits?
MDSAP audits are conducted by authorized Auditing Organizations (AOs), which are entities designated and approved by the regulatory authorities participating in the program. Like Notified Bodies in the EU, these AOs operate under strict guidelines and are equipped with the expertise to assess compliance with the applicable regulatory requirements.
Manufacturers must select an AO based on their specific needs and geographical distribution. Choosing an AO with experience in the relevant regulatory jurisdictions is crucial to ensure a thorough and accurate evaluation during the audit process. Choosing your AO niche and ensuring it matches your own can make or break your audit.
How's the MDSAP audit process, and what does it involve?
The MDSAP audit process is structured to assess a medical device manufacturer's quality management system (QMS) against the requirements of the participating regulatory authorities. The audit covers various elements, including management responsibility, resource management, product realization, measurement, analysis, and improvement.
During the audit, the AOs review documentation, conduct interviews and assess the implementation of the manufacturer's QMS. The audit process is comprehensive and systematic, covering all facets of the organization's operations related to developing, manufacturing, and distributing medical devices.
MDSAP incorporates a risk-based approach, which allows the AOs to tailor the audit scope based on the manufacturer's specific product portfolio and the associated regulatory requirements. This ensures that the audit is efficient and focused on areas of higher risk.
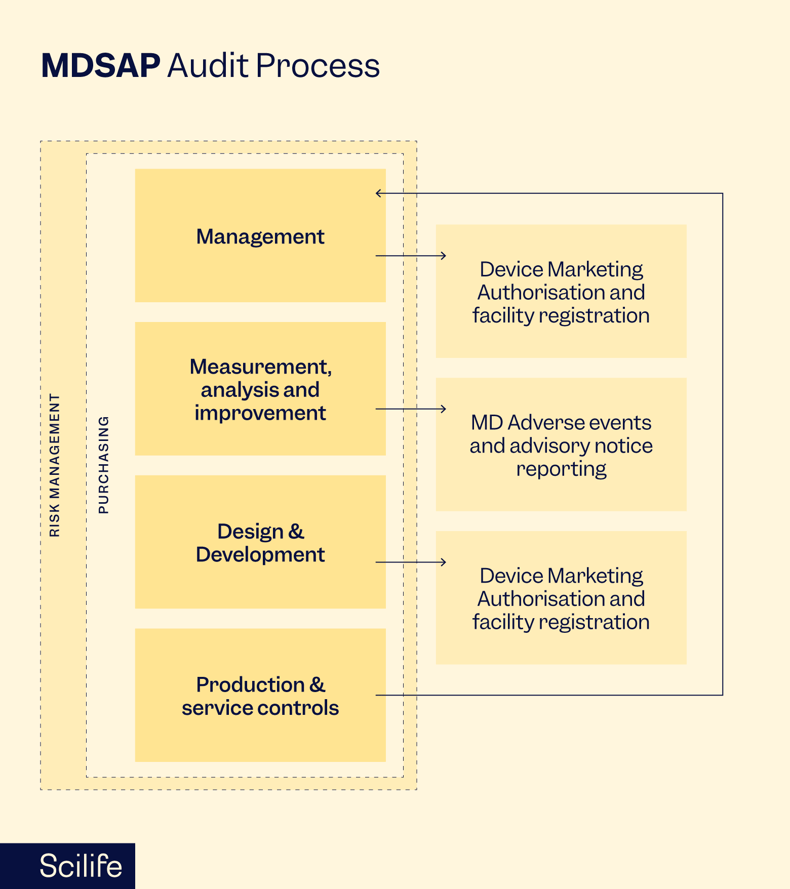
How to prepare for MDSAP audits
There is one all-encompassing key to a successful MDSAP audit: adequate preparation. The best way for manufacturers to ensure a successful audit is to adopt a proactive approach to ensure compliance with regulatory requirements and streamline the audit process.
There are three essential steps to prepare for an MDSAP audit:
- Understand the regulatory requirements: before embarking on the MDSAP journey, thoroughly understand the regulatory requirements of each participating country. This includes familiarizing yourself with the applicable standards, regulations, and guidelines to ensure compliance.
- Perform a gap analysis: conduct a comprehensive gap analysis of your existing quality management system against MDSAP requirements. Identify areas that may require improvement or modification to align with the program's criteria.
- Implement a robust eQMS: establishing an electronic Quality Management System (eQMS) is crucial for efficient MDSAP preparation. An eQMS helps manage documentation, track processes, and demonstrate compliance in a centralized and accessible manner.
What's the role of an eQMS in MDSAP audits?
An eQMS plays a pivotal role in MDSAP preparation and ongoing compliance. It is a centralized platform for managing documentation, processes, and quality-related activities.
Here's how an eQMS contributes to MDSAP readiness:
- Easier document management: an eQMS facilitates the organization and control of critical documents, including policies, procedures, and records. This ensures that documentation is up-to-date, accessible, and aligned with MDSAP requirements.
- Process automation: automation of critical processes within the QMS streamlines operations and reduces the risk of human errors. This is particularly beneficial in demonstrating compliance during the MDSAP audit.
- Real-time monitoring: an eQMS enables real-time tracking of quality-related metrics and performance indicators. This proactive approach allows manufacturers to address potential issues before they escalate and ensures continuous improvement.
- Audit management: the audit management module of an eQMS helps with planning, conducting, and documenting internal audits. This prepares the organization for the MDSAP audit by instilling a regular self-assessment and improvement culture.
- Training and competence management: ensuring that personnel are adequately trained and competent is fundamental to MDSAP compliance. An eQMS streamlines training management, tracking employee competencies, and ensuring that training records are readily available during audits.
Benefits of MDSAP
We've discussed MDSAP at length. But what kind of benefit can medical device manufacturers gain from participating in MDSAP? Participating in the MDSAP offers several advantages for medical device manufacturers:
Reduced Regulatory Burden
MDSAP simplifies the audit process by allowing manufacturers to undergo a single audit to meet the requirements of multiple regulatory authorities. This reduces the overall regulatory burden, streamlining the compliance process.
Global Market Access
Successful participation in MDSAP gives manufacturers a competitive edge by facilitating access to major international markets. This is particularly valuable for companies looking to expand their reach and market their products globally.
Improved Audit Quality
MDSAP's collaborative approach ensures that audits are conducted consistently and to a high standard. This enhances the quality of audits, providing manufacturers with valuable insights and opportunities for continuous improvement.
Enhanced Risk Management
MDSAP's risk-based approach allows manufacturers to focus on areas of higher risk, ensuring that resources are allocated effectively. This proactive risk management strategy contributes to medical devices' overall safety and efficacy.
Get a comprehensive overview of how to build a proper Medical Device Quality Management System by watching the video below:
Conclusion
In conclusion, preparing for the Medical Device Single Audit Program (MDSAP) is a strategic imperative for medical device manufacturers aiming to navigate the complex landscape of global regulatory compliance. By understanding the program's purpose and the entities involved and implementing a robust preparation strategy, manufacturers can meet regulatory requirements and gain a competitive advantage in the global market.
The role of an electronic Quality Management System (eQMS) must be balanced in this process. An eQMS facilitates efficient preparation for MDSAP audits and supports ongoing compliance and continuous improvement. Embracing the benefits of MDSAP positions manufacturers to thrive in an increasingly interconnected and regulated medical device industry.
Discover how Scilife Smart QMS for Medical Devices can help you prepare for the MDSAP Audits!