Having the FDA knocking on your door for a medical device inspection is scary! However, the management of FDA inspections for medical devices is a critical aspect of ensuring compliance with regulatory standards and maintaining the integrity of the medical device industry. The US Food and Drug Administration (FDA) is responsible for safeguarding public health by regulating medical devices and medical device systems and ensuring manufacturers are playing by the rules.
An FDA inspection is a thorough examination conducted by the agency to keep an eye on the medical device manufacturer's compliance with regulatory requirements.
In this article, we will explore the different types of FDA medical device inspections and their processes, and strategies for ensuring inspection readiness, and spill the beans on our best practices to navigate these inspections successfully.
Want to learn how to build and maintain a powerful QMS that stands up to FDA inspections every time? Our QARA guide to medical device quality management systems walks you through the essentials to get it right from the start.
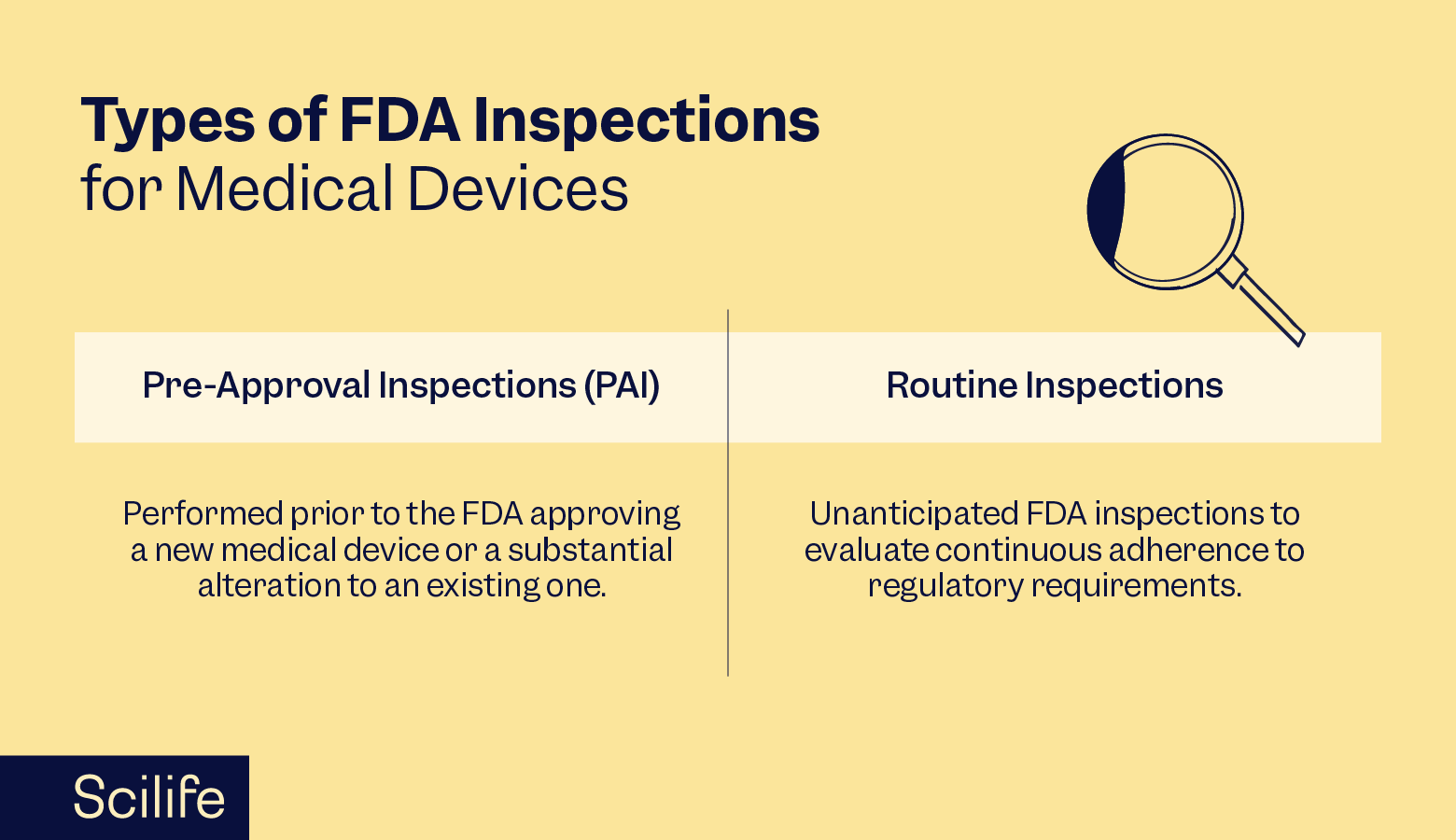
Types of FDA Medical Device Inspections and their processes
FDA medical devices inspections come in various forms, each serving a specific purpose. The two primary types are pre-approval inspections (PAI) and routine inspections.
Pre-Approval Inspections (PAI): Pre-approval inspections are conducted before the FDA approves a new medical device or a significant modification to an existing one. Think of it as the FDA making sure you know what you’re doing before they give you the okay to start mass-production. The inspection focuses on ensuring that the manufacturing processes, facilities, and controls comply with regulatory standards. Manufacturers must be well-prepared for PAI to demonstrate the safety and efficacy of their products and to ensure successful inspections, leading to approval of their device or device modification.
Routine Inspections: Routine inspections, also known as surveillance inspections, are unannounced visits by the FDA to assess ongoing compliance with regulations. These inspections can occur at any time, placing a premium on maintaining a state of continuous readiness. It’s like a pop quiz for your company. During routine inspections, the FDA reviews various aspects, including quality control, manufacturing processes, documentation, and adverse event reporting.
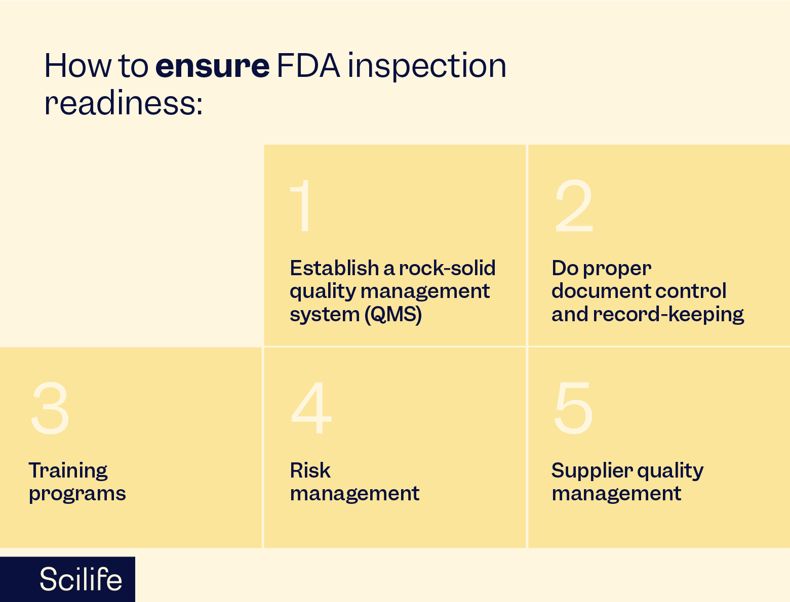
How can we ensure inspection readiness?
To prepare for FDA inspections and maintain compliance, medical device manufacturers must adopt proactive measures. Here are five key strategies for ensuring inspection readiness:
Establish a rock-solid quality management system (QMS): A well-defined QMS is the foundation of regulatory compliance. It should cover all aspects of the device lifecycle, including design, manufacturing, testing, and post-market surveillance. Update it regularly to align with changing regulations and industry standards.
Do proper document control and record-keeping: Maintaining meticulous documentation of all processes, procedures, and activities related to medical device manufacturing is your golden ticket. Implement a comprehensive document control system to manage versions and revisions effectively. Accurate and up-to-date records facilitate transparency during inspections.
Training programs: Train personnel at all levels on regulatory requirements, company policies, and the importance of compliance. A knowledgeable and well-trained workforce is crucial for maintaining consistent adherence to quality standards.
Risk management: Implement a robust risk management process to identify, assess, and mitigate potential risks associated with medical devices. This should include comprehensive risk assessments and strategies for minimizing or eliminating identified risks.
Supplier quality management: Ensure that suppliers and contractors adhere to the same high-quality standards as your organization. Regularly audit and assess supplier performance to maintain a reliable supply chain. Your supply chain is only as strong as its weakest link.
Best Practices and Tips to Go Through an Inspection
Facing an FDA medical device inspection, especially an unannounced and unpredictable one, might sound nerve-wracking, but with careful preparation and adherence to best practices, manufacturers can navigate the process successfully.
Here are our key best practices and tips to add to your inspection cheat sheet:
Develop an FDA inspection checklist: Create a detailed FDA inspection checklist based on regulatory requirements and past inspection experiences. This checklist should cover all aspects of the inspection, from facility walkthroughs to document reviews.
Practice makes perfect: Conduct regular mock inspections to simulate the real inspection process. This helps identify areas of weakness and allows the organization to address issues before an actual inspection occurs. Get all your teams together to participate in a comprehensive evaluation – it’s the best way to identify your weak spots and run a tight ship going forward.
Designate an inspection response team: Establish a dedicated team responsible for managing the inspection process. This team should include individuals from various departments, such as quality, regulatory affairs, and manufacturing. Spend some extra time making sure everyone knows their roles and responsibilities.
Make your documentation accessible: Have all relevant documentation readily accessible during an inspection. This includes standard operating procedures (SOPs), batch records, testing protocols, and quality records. Well-organized documentation expedites the inspection process and demonstrates transparency.
Maintain open communication with inspectors: The FDA wants you to do well, and they want to ensure painless inspections. Foster a collaborative and transparent relationship with the FDA inspectors and address their queries promptly with clear and concise explanations. Being friendly and cooperating can positively influence the outcome of the inspection.
Work on continuous improvement: Implement a culture of continuous improvement within the organization. Regularly assess and enhance processes based on internal audits, corrective and preventive actions (CAPA), and lessons learned from previous inspections.
Conclusion
Navigating an FDA medical device inspection can be stressful – but inspections are a complex but essential aspect of ensuring regulatory compliance and maintaining the trust of patients and healthcare professionals. By understanding the types of inspections, you can potentially face, preparing for them diligently, and adopting best practices, you can streamline the inspection process and mitigate potential risks.
The journey towards inspection readiness involves establishing robust quality management systems, comprehensive training programs, and effective risk management strategies. Regular mock inspections and the development of a thorough FDA inspection checklist contribute to the overall preparedness of an organization.
Ultimately, successful navigation of FDA inspections requires a proactive and collaborative approach, emphasizing open communication, transparency, and a commitment to continuous improvement.
As the medical device industry evolves, staying informed about regulatory changes and industry trends is crucial. By adhering to best practices and embracing a culture of compliance, manufacturers can not only pass FDA inspections but also contribute to the overall safety and efficacy of medical devices and, ultimately, patients.
Discover how a Smart QMS can help you go through any FDA inspection effortlessly.