The prime directive of medical device manufacturers is the continued safety of patients through safe and effective medical devices. A critical aspect of medical device safety is ensuring regulatory compliance of any medical device destined for the commercial market. Design controls play a pivotal role in achieving regulatory compliance by providing a systematic framework for managing the design and development process of medical devices and in vitro diagnostics devices.
This article delves into the intricacies of these design controls for the medical device industry, exploring their purpose, key components, regulatory requirements, and practical tips for implementation.
If you want to see how design controls fit into the bigger quality management picture, our QARA guide to medical device quality management systems is a great place to start!
So, what are design controls for the medical device industry, and why do we need them?
Design controls refer to a set of systematic procedures implemented throughout the design and development lifecycle of medical devices. Their primary purpose is to ensure that devices are safe, effective, and meet the intended user needs and regulatory requirements. By incorporating design controls throughout the lifecycle of their medical devices, manufacturers can mitigate risks, maintain quality, and continuously improve their devices.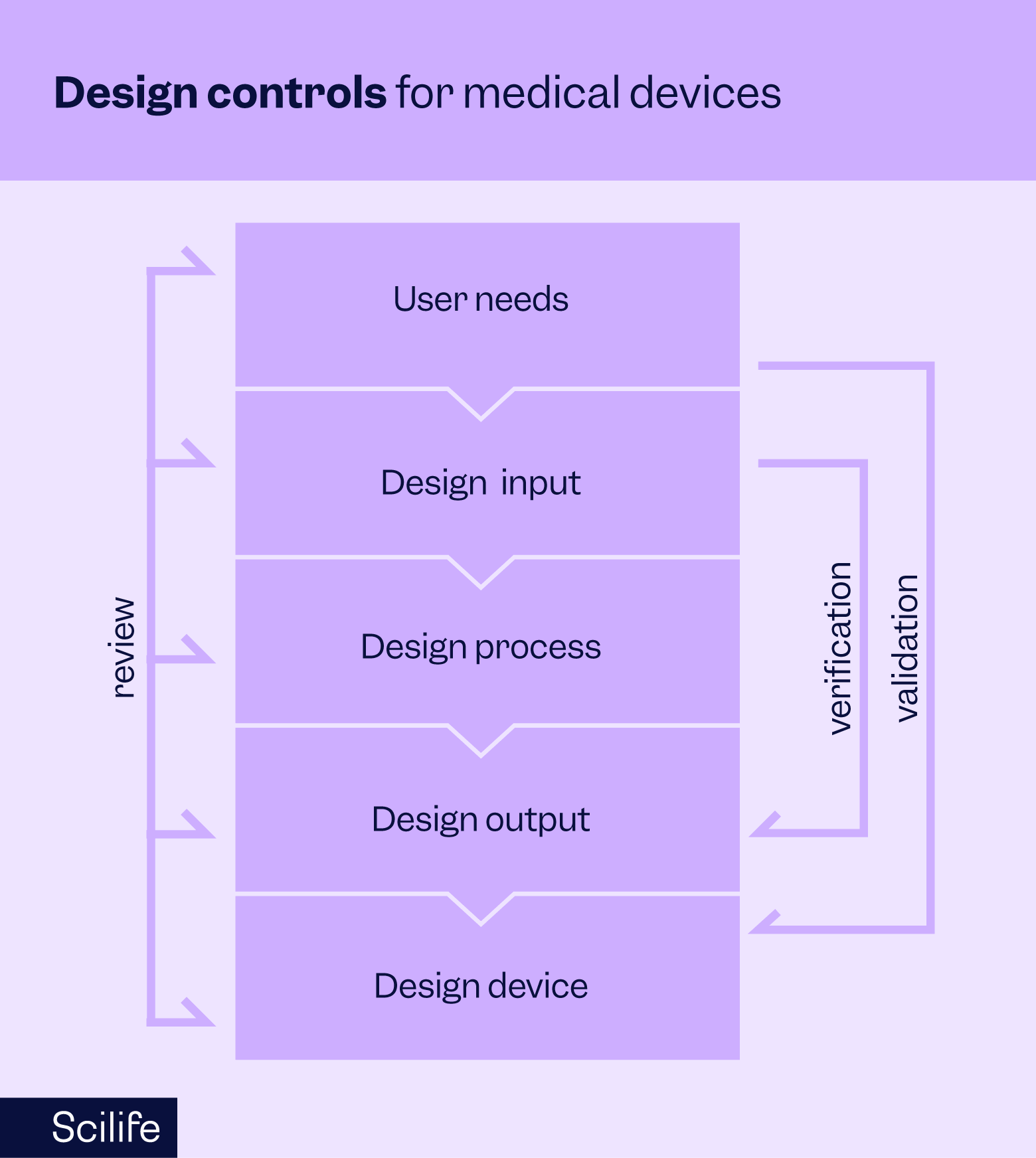
Here's an explanation of each design control:
Design and development planning:
Planning is where it all begins!
The planning phase establishes the strategy and structure for the entire design and development process.
It includes defining responsibilities, setting timelines, allocating resources, identifying interdepartmental interfaces, and specifying the criteria for design reviews.
Design plans should be scalable and adaptable to changes throughout the project lifecycle.
A well-maintained plan helps maintain compliance, ensures design traceability, and supports efficient risk and resource management.
Design input
Design inputs are the documented physical, functional, performance, safety, and regulatory requirements that a device must meet.
Sources include user needs, clinical considerations, intended use, applicable standards (e.g., IEC 60601, ISO 14971), and risk management outputs.
Design inputs must be complete, unambiguous, and measurable wherever possible.
Inputs are subject to design review and must align with regulatory expectations.
This phase also links directly to risk management; identified hazards must be translated into input requirements where appropriate.
Inputs ultimately form the basis for verification testing.
Design output
Design outputs are the results of design activities, such as engineering drawings, component specifications, manufacturing instructions, inspection criteria, and software code.
Outputs must be defined in a way that enables consistent product realization and should include acceptance criteria.
Outputs must be traceable to design inputs and conform to the device specifications required for production.
The QMS requires that these outputs be reviewed and verified before proceeding to manufacturing. Outputs also contribute to the Device Master Record (DMR), which serves as the authoritative reference for manufacturing and quality control.
Design verification
Design verification ensures that design outputs correctly implement the design inputs. It involves objective evidence obtained through inspection, analysis, demonstration, or testing. For example, verifying that a thermometer displays the correct temperature range as defined in the inputs.
Verification protocols and reports must be documented, reviewed, and approved.
The QMS mandates traceability between test results and specific input requirements. If verification fails, corrective action must be taken and documented through established change control procedures.
All verification activities contribute to the Design History File (DHF).
Design validation
Design validation confirms that the final product meets user needs and intended use under actual or simulated conditions. It is performed on initial production units, lots, or batches. Validation activities may include usability testing (per IEC 62366), human factors evaluations, and clinical trials.
Your QMS requires that validation is conducted under defined, documented protocols with acceptance criteria and risk considerations. Validation must be based on user needs and typically includes risk mitigation verification.
Results are recorded in the DHF and are crucial for regulatory submissions and product approvals.
Design review
Design reviews are planned, formal evaluations of design progress. They assess whether design inputs are being met, identify issues or inconsistencies, and determine whether the project is ready to proceed to the next phase.
Each review includes independent reviewers who were not directly involved in the development under review.
Design reviews must be documented, including attendees, issues raised, and action items.
The QMS ensures these reviews are scheduled at appropriate stages and that follow-up actions (e.g., design changes, additional testing) are tracked and closed.
Reviews help maintain regulatory compliance and manage risk proactively.
Design transfer
Design transfer is the formal process of moving a product from design into manufacturing. This includes finalizing specifications, bills of materials (BOMs), assembly procedures, and inspection protocols. The goal is to ensure the product can be manufactured repeatedly and reliably.
All documents required for production—such as the DMR—should be reviewed. Any gaps between design and production (e.g., material selection, tooling capability, process validation) must be resolved.
Transfer activities may also trigger updates to training materials, quality plans, and supplier documentation.
Design changes
This phase manages modifications to the design throughout development and post-market. Changes might arise from test results, design reviews, regulatory feedback, supplier changes, or post-market surveillance. The goal is to maintain product integrity without introducing new risks.
All changes must go through formal change control per QMS procedures. This includes impact assessments, design re-verification/validation (if needed), and documentation updates (including DHF, DMR, and labeling).
The change process helps ensure continued regulatory compliance, product safety, and performance.
Design History File (DHF)
The DHF is a compilation of all records that show the device was developed according to the approved design plan and regulatory requirements.
It includes documentation from all phases: planning, inputs, outputs, reviews, verification, validation, and changes.
The DHF supports internal audits, inspections, and regulatory submissions which is why it should be maintained throughout development and easily accessible for review. It’s the evidence that your design process was controlled, compliant, and thoroughly documented.
Recommended learning: Your step-by-step guide to bringing a medical device to market
Design controls according to FDA 21 CFR 820 vs. ISO 13485
So now that we have the device controls for the medical device industry down, how are they presented in the two major quality system regulations?
FDA 21 CFR 820:
The FDA's Quality System Regulation (QSR) outlines specific requirements for design controls in the United States, emphasizing documentation, traceability, and risk management throughout the design process. Compliance with FDA regulations is essential for market approval, quality, and safety. Not to mention, FDA compliance is mandatory for any medical device manufacturer operating in the US.
ISO 13485:
The international standard for quality management systems in the medical device industry guides design control processes, emphasizing a risk-based approach, lifecycle management, and alignment with regulatory requirements worldwide. While not mandatory, ISO 13485 certification is the most frequently used QMS standard in the EU and demonstrates a manufacturer's commitment to quality and regulatory compliance on a global scale.
How do design controls ensure traceability?
Design controls ensure traceability by creating clear, documented links between every stage of medical device development—connecting user needs, design inputs, outputs, verification, validation, and changes in a cohesive, auditable way.
It begins with well-defined design inputs, such as user needs, performance specifications, and regulatory requirements. These inputs are directly tied to design outputs like engineering drawings, software, and material specifications. This linkage ensures the final design delivers on its original intent.
Verification confirms that outputs meet inputs, while validation ensures the final product fulfills its intended use under real-world conditions. Each test or evaluation is documented and mapped back to its corresponding requirement, maintaining traceability throughout.
The Design History File (DHF) serves as a central record of the entire process, showing what was designed, why, how it was tested, and how decisions were made. Any design changes go through formal change control, with updates to all related documents and, if needed, re-verification or re-validation—keeping traceability intact even as the design evolves.
Ultimately, design controls make it possible to track each feature or function of a medical device back to its source requirement, demonstrating compliance, enabling effective quality management, and simplifying audits or investigations.
What is a Design Control Traceability Matrix?
A Design Control Traceability Matrix (DCTM) is a tool that maps design inputs—such as user needs and regulatory requirements—to their corresponding outputs, verification and validation activities, and sometimes risk controls. It ensures that every requirement is implemented, tested, and traceable throughout the development process.
The matrix usually takes the form of a table, with each row representing a specific requirement. Adjacent columns show how that requirement is addressed in the design, how it’s verified (e.g., via bench testing), validated (e.g., through usability studies), and, if applicable, linked to a risk control measure.
This document plays a key role in the Design History File (DHF) and is often reviewed during audits. It demonstrates that the design process is complete, controlled, and compliant with regulatory standards like FDA 21 CFR 820.30 and ISO 13485. It also supports change management by making it easy to trace the impact of design changes across related elements.
In short, the traceability matrix ties everything together—requirements, implementation, and testing—into a clear, auditable framework.
Tips for solid design controls for medical device industry
- Clear documentation: To ensure traceability and compliance, maintain thorough documentation throughout the design process, including design inputs, outputs, reviews, and changes. Document control procedures should facilitate version control, approvals, and access to relevant stakeholders.
- Cross-functional collaboration: Foster collaboration among multidisciplinary teams, including engineers, clinicians, regulatory experts, and quality professionals, to incorporate diverse perspectives and expertise. Regular communication and stakeholder engagement enhance transparency and alignment with user needs and regulatory requirements.
- Risk management: Integrate risk management activities into the design process, identifying and mitigating potential hazards and ensuring that residual risks are acceptable. Risk management tools like Failure Mode and Effects Analysis (FMEA) help prioritize risks and implement appropriate controls to minimize adverse events.
- Validation testing: Conduct comprehensive validation testing to confirm that the device meets user needs and performs safely and effectively in real-world conditions. Validation protocols should be well-defined and incorporate relevant test methods, acceptance criteria, and statistical analysis to provide meaningful results.
- Continuous improvement: Embrace a culture of constant improvement, solicit feedback from users, monitor post-market performance, and proactively address any issues or opportunities for enhancement. Post-market surveillance and feedback mechanisms enable manufacturers to identify trends, address emerging risks, and enhance product quality over time.
Key takeaways
Here are three key takeaways about design control to sum it all up:
- Design controls create a structured, documented process that guides medical device development from initial concept to final product, ensuring all requirements are met systematically.
- Traceability is central—design controls link user needs, technical specifications, verification, validation, and changes, providing a clear audit trail that demonstrates compliance and product safety.
- Integration with your QMS ensures quality and compliance by embedding design controls into your company’s procedures, enabling effective risk management, controlled change processes, and readiness for regulatory inspections
How an eQMS can help you with design controls
An electronic Quality Management System (eQMS) can streamline and automate many aspects of design controls for the medical device industry to facilitate documentation, collaboration, and compliance. Key features usually include document control, change management, risk management, validation tracking, and audit trails, enabling efficient design process management and reducing the administrative burden on teams. An eQMS achieves this by providing:
- A centralized management of all design control activities in one unified platform
- Enhanced visibility into every stage of the design process
- Complete traceability across documents, decisions, and changes
- Real-time access to critical data throughout the entire product lifecycle

Design controls are foundational to developing safe, effective, and compliant medical devices. By adhering to rigorous processes and standards, manufacturers can navigate regulatory requirements, mitigate risks, and ultimately deliver products that improve patient outcomes and enhance healthcare delivery.
Embracing best practices, leveraging technology solutions, and fostering a culture of quality and innovation are essential in achieving success in today's dynamic medical device industry.
Discover how Scilife Smart QMS for medical devices can help you stay fully compliant.