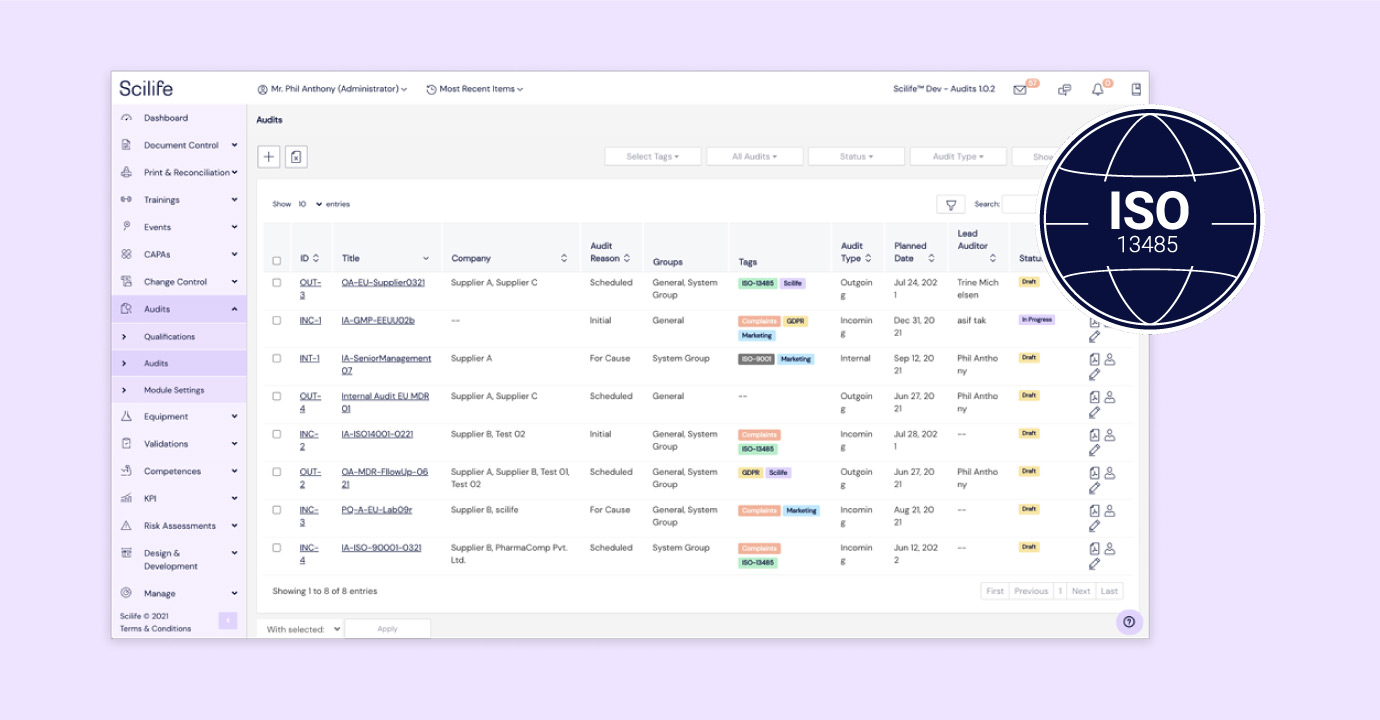
Medical device teams often spend months perfecting a breakthrough design. But the moment ISO 13485 certification and compliance enter the picture, momentum often grinds to a halt.
The quality team has been brought in too late. Documents are scattered, training records are outdated, CAPAs live in spreadsheets, and the audit clock is already ticking.
Suddenly, compliance feels less like a safety net and more like a brake pedal.
But it doesn’t have to. Imagine an ISO 13485 QMS software that flags skipped steps instantly, locks down deviations, and surfaces risks before they snowball. Less firefighting. Stronger trust.
That’s where Scilife changes the story. With our ISO 13485 QMS software, backed by robust ISO 13485 QMS software validation, compliance becomes more than just a regulatory checkbox. It becomes a driver of speed, efficiency, and trust.
In this article, we’ll dive into exactly how cloud QMS software for ISO 13485 compliance transforms chaotic processes and regulatory nightmares into a streamlined, smooth, inspection-ready process and show you where Scilife wins where others fall short.
Let’s crack on!
Why ISO 13485 compliance is non-negotiable (and why specialized QMS software matters)
FDA clearance, ease of use, affordable price tag, a doctor’s recommendation—brands get picked for many reasons.
But at the core, it all boils down to trust in the quality behind the product.
And that’s exactly where ISO 13485, the international gold standard for medical device quality management, steps in to ensure you can deliver on that promise every time.
Patients and providers don’t buy your device. They’re buying the assurance that it was built, tested, and delivered with care and compliance.
The reality is that in our digital world, outdated, paper-based systems and clunky legacy tools are not enough to get the job done right. They create complexity and unnecessary risk that can make the difference between smooth market entry and costly delays.
Medical device recalls are on the rise—and they highlight cracks in the very systems ISO 13485 QMS software is meant to safeguard. ISO 13485 mistakes such as weak design controls, sloppy documentation, and poor risk management are exactly what specialized solutions like Scilife prevent.
That’s why a solid ISO 13485 QMS software like Scilife, supported by proper ISO 13485 QMS software validation is no longer a nice-to-have. It locks down processes so recalls don’t happen in the first place.
Medical device recalls in 2024
- In the US alone, device recalls surged to 1,059 in 2024 (an 8.6% increase from 2023), affecting over 440 million devices.
- Class I devices also hit a 15% year-high number of recalls.
- And the number 1 reason for device recalls? Device failure, followed by quality, mislabelling, software, and parts issues.

These numbers point to cracks in the very systems ISO 13485 is designed to safeguard: weak design controls, poor risk management and validation, sloppy document handling, and delayed responses to quality events.
That’s why solid and GAMP 5 validated ISO 13485 QMS software like Scilife matters is no longer just a nice-to-have.
It locks down those processes so recalls don’t happen in the first place.
QMSR countdown: Why ISO 13485 compliance can’t wait
With the FDA’s new Quality Management System Regulation (QMSR), 21 CFR Part 820 will fully align with ISO 13485:2016 starting February 2, 2026. That means that if your quality system isn’t already built around ISO 13485, the clock is ticking. ISO 13485 isn’t just best practice anymore; it will be the law.
QMSR keeps the same intent as the old QSR, but there’s no more room for shortcuts. Under QMSR, FDA inspections will more closely mirror ISO 13485 audits. Records such as internal audits, supplier evaluations, and management reviews will face closer scrutiny. In other words, compliance can’t be a quick fix, and there will be nowhere to hide weak processes or poor documentation.
Your QMS needs to run on ISO 13485 principles from the ground up.
This is why medical device companies are moving to cloud QMS software for ISO 13485 compliance—it embeds compliance into daily work instead of treating it like a separate task.
The right ISO 13485 QMS software makes compliance part of how you work every day, so you’re always ready for inspections, instead of scrambling when the auditor arrives.
Scilife helps medical device companies build safer, better products with confidence and speed.
We partner with medical device pioneers like Biocartis, Novosanis, Amnovis, and Shoebox to digitalize quality and compliance. Our scalable QMS platform centralizes processes, ensures GxP compliance, and grows with you—making it simple to meet standards from ISO 13485 to FDA requirements.
How Scilife’s ISO 13485 QMS software makes compliance effortless
Scilife QMS software for medical devices was designed to help medical device and In Vitro Diagnostic (IVD) organizations like yours meet ISO 13485 requirements with less friction and more confidence.
Our customers benefit from:
- ISO 13485:2016 compliance by design – integrated risk management, audit trails, and e-signatures aligned with FDA 21 CFR Part 11 and EU GMP Annex 11.
- Smart document and records control – versioning, metadata, approvals, and traceability all in one place.
- Automated training and competence tracking – role-based training with reminders, notifications, and retraining triggers.
- Predefined workflows for quality processes – CAPAs, change control, risk assessments, audits, supplier management, and more.
- Accelerated design and development – design controls, validation, and streamlined approvals to cut time-to-market by up to 50%.
- Equipment and supplier management – stay inspection-ready with calibration tracking and ensure supplier compliance with audits, risk assessments, and quality agreements.
- Data integrity and security – barcode reconciliation, audit trails, and GAMP5-validated AWS hosting safeguard your records and processes.
Scilife is not only natively aligned with ISO 13485:2016 but also with 21 CFR Part 11, EU GMP Annex 11, and GAMP5 validation principles. That means every workflow, every document, and every audit trail is ready to stand up to regulatory scrutiny.
Build as a cloud QMS software for ISO 13485 compliance, it centralizes your documentation, automates training and reduces the steps you have to take to complete a quality process.
What this means for you: no scrambling to prepare when inspectors arrive. Compliance is built into your daily operations.

How our QMS software ensures compliance with ISO 13485 clause by clause
Here’s a breakdown of how our ISO 13485 QMS software helps you comply with each clause of ISO 13485:

Clause 4 – Quality Management System (QMS)
ISO 13485 requires organizations to establish and maintain a documented QMS.
- Scilife provides a digital QMS software where your quality manual, policies, and objectives are always current and accessible.
- Our document control tool ensures versioning, metadata, and approval cascades are automated and traceable.
- Compliant e-signatures and audit trails meet ISO 13485, 21 CFR Part 11, and EU GMP Annex 11 requirements.
- Print & Reconciliation with barcodes goes beyond ISO 13485 requirements to strengthen data integrity and prevent unauthorized document use.
- Automated periodic reviews and reminders keep all documentation accurate, relevant, and inspection-ready.
Clause 5 – Management Responsibility
Top management must demonstrate leadership, commitment, and ongoing review of the QMS.
- We give leadership real-time dashboards and KPIs so they can see how the QMS is performing at a glance.
- Thanks to configurable workflows and process monitoring, we make decision-making evidence-based, not guesswork with configurable workflows and process monitoring.
- Because quality should be embraced, not enforced, we also use learning tools such as the Scilife Academy to help your teams feel engaged and part of the process.
- Clear role assignment and accountability strengthen oversight and compliance.
Clause 6 – Resource Management
ISO 13485 requires that your team is properly trained, your equipment and facilities are well maintained, and your work environment enables consistent product quality.
- Scilife ensures staff competence with role-based training, competence matrices, and CV management.
- Automatic reminders and retraining notifications keep qualifications current and audit-ready.
- Certificates and skills tracking provide clear proof of compliance for inspectors.
- Flexible training supports online courses, external sessions, videos, and effectiveness checks.
- Managers gain full visibility of employee competence, ensuring the workforce remains qualified and compliant.
- Our equipment management solution makes it simple to plan, record, and demonstrate calibration and maintenance tasks — giving you confidence that your infrastructure is always inspection-ready.
Clause 7 – Product Realization
From design to delivery, every step of making a medical device must be planned, controlled, and documented to ensure safety and effectiveness.
- Scilife streamlines design and development with full traceability across every requirement, review, and approval. We help customers reduce time-to-market by up to 30%!
- Our risk assessment workflows (with built-in FMEA) connect directly to deviations, CAPAs, and audits, so risks are never overlooked.
- Our supplier management solution supports qualification, audits, and risk-based supplier evaluation in line with MDR and ISO 13485.
- Events, CAPAs, and change control workflows ensure every deviation, complaint, and change is documented, investigated, and resolved.
- Collaboration tools eliminate silos and promote end-to-end visibility across product realization.
Clause 8 – Measurement, Analysis, and Improvement
Organizations must monitor processes, conduct audits, and drive continual improvement.
- Our audit management solution simplifies internal, external, and supplier audits, with findings linked to CAPAs and quality events.
- Audit results link directly to CAPAs and risk assessments, ensuring traceability.
- CAPA workflows include effectiveness checks, ensuring preventive and corrective actions are fully closed.
- With real-time analytics, reports, and KPI dashboards, you’ll always have the insights you need to improve your processes and strengthen compliance.
Where Scilife wins where others fall short
There are plenty of eQMS vendors that promise ISO 13485 compliance. But Scilife goes further! Our ISO 13485 QMS software is intuitive, scalable, and built with analytics and training tools that make compliance not just easier—but smarter.
Intuitive and easy to use
Unlike many rigid, form-heavy QMS solutions, Scilife is designed with the user in mind.
Our clean, modern interface makes it easy for anyone, from quality professionals to executives, to find information, complete tasks, create documents, and stay compliant without extra training or headaches.
Adoption is natural, engagement is higher, and your quality culture grows stronger!
Scalability advantage
Other systems lock you into rigid setups. Scilife adapts to your size and stage.
Whether you’re a fast-moving startup or an established enterprise, our design and flexible pricing mean your QMS grows as you grow without disruption.
Free quality training
Most systems stop at compliance. Scilife invests in your people.
With Scilife Academy, we provide a dedicated free hub for continuous learning where QA/RA professionals can take courses and certifications on quality trends, regulations, and best practices.
Smarter decisions with advanced analytics
Compliance is just the baseline. Scilife’s analytics, custom reporting, trend analysis, and benchmarking give you deep insights into your processes and performance. You’ll make faster, smarter decisions that drive real improvement across your organization.
"We did a cost-benefit analysis, and we really like how everything is connected on Scilife. It makes it easy to produce an audit trail and access necessary information quickly."
Athanasios Batagiannis, General Manager
ISO 13485 QMS software validation
Software validation is a key requirement for ISO 13485:2016.
Every application that supports your QMS must be validated before you use it, and again whenever significant changes are made.
The purpose is to prove that your software works as intended, reliably and consistently, so you can trust it during audits and daily operations.
ISO 13485 requires that QMS software be validated before use and after significant changes. We follow a GAMP5 validation approach on the secure Amazon Web Services (AWS) platform. This means that our ISO 13495 QMS software is pre-validated for standard functionality and workflows in an independent environment, reducing the burden on your team.
If your processes align with Scilife’s default workflows, you can immediately benefit from this validated environment.
For added assurance, we provide a complete validation package with all documentation you need — making it easy to demonstrate compliance, maintain data integrity, and satisfy regulatory expectations without unnecessary stress. Other vendors don't make ISO 13485 QMS software validation this easy!
Recommended learning: Validation doesn’t have to slow you down. This guide shows you how to keep compliance tight while moving fast with Scilife.
Conclusion: Ready to make ISO 13485 compliance effortless?
Here at Scilife, we believe in taking quality beyond a 'cost-of-doing-business' perspective.
Our mission is to make quality easy for everyone — not only QA teams, but also product, R&D, and every department that plays a role in bringing safe and effective devices to market.
By giving your teams intuitive ISO 13485 QMS software and instant access to data, Scilife makes quality a natural part of everyday work, not to mention that our ISO 13485 QMS software validation comes straight out the box!
From our customer success stories, we know when everyone takes ownership of quality, compliance becomes effortless. The result is a stronger culture, fewer risks, faster product development, and full confidence that your business is always audit-ready.
And of course, all while meeting ISO 13485 requirements with confidence!