In the medical device industry, everything starts with a solid design and development plan. As a QA or RA professional, your deep understanding of this plan is crucial.
Regulators don't just want to see a finished medical device—they need clear evidence of how it was designed, developed, and tested against mandatory quality standards. Your knowledge and expertise in this area ensure every device placed on the market is safe, effective, and fit for purpose.
If you work in quality, compliance, or regulatory affairs, you already know how much depends on getting this right. A well-structured design and development plan not only keeps you compliant but also makes audits smoother, builds trust with health authorities, and, most importantly, protects patients.
In this post, I’ll explain why a medical device design and development plan is so important, walk through the key steps to creating a plan that auditors will actually value, and share practical suggestions for meeting regulatory requirements with a robust Quality Management System (QMS) specifically tailored for medical device design and development.
Whether you're refining existing processes or building a plan from scratch, this guide will help you align with industry standards and demonstrate that your medical device is backed by rigorous, transparent development practices.
Key takeaways
What is a medical device design and development plan?
A medical device design and development plan is more than just a project outline—it’s a regulatory requirement and a cornerstone of patient safety. Both ISO 13485 and FDA 21 CFR Part 820 mandate that manufacturers document how a device will be designed, developed, and brought to market.
Key to getting your design and development plan right is remembering: it’s not static; it’s a living document that evolves as the project progresses, requiring regular review, updates, and approvals.
This approach allows for adaptability to changing project needs and continuous improvement, which are key to meeting regulatory requirements. A medical device design and development plan defines the objectives of the product, maps out development activities, and assigns responsibilities across teams.
It ensures that critical inputs—from engineering and quality to regulatory and risk management—are captured and aligned. For simple products, the plan may be as straightforward as a flow chart, while complex devices often demand a detailed Gantt chart with multiple work streams.
Ultimately, a strong design and development plan does more than satisfy auditors. It creates structure, ensures accountability, and provides the evidence regulators need to confirm that your device is safe, effective, and built to the highest quality standards.
Don’t underestimate the value of the right tools. Leveraging Scilife’s bespoke medical device design and development software can eliminate rework, prevent delays, and clear away the messy workflows that often hold teams back!
What are regulatory expectations for a medical device design and development plan?
ISO 13485 design and development plan
ISO 13485
is the globally harmonized quality management standard for medical devices, setting out what manufacturers must do to ensure their products are consistently safe, effective, and compliant.
When it comes to design and development, the standard requires manufacturers to create and maintain an ISO 13485 design and development plan that guides the entire process—from defining objectives and responsibilities to integrating risk management activities.
Remember! The plan is not just a one-time exercise.
Your ISO 13485 design and development plan has to evolve in tandem with the project and provide the documented evidence that regulators and auditors expect, and it needs to be reviewed, updated, and approved as the project progresses.
Key expectations of an ISO 13485 design and development plan include documented procedures to control and validate every stage of the lifecycle, ensuring that any process not fully verified by inspection performs as intended.
Comprehensive documentation and traceability are central: records must demonstrate how design activities align with broader QMS processes, including risk management, supplier oversight, and corrective and preventive action (CAPA).
By embedding design planning within the QMS, ISO 13485 ensures that quality, safety, and regulatory requirements are built into the device from day one.
Recommended learning: Struggling with design controls? Here’s a practical guide every medical device professional should read.
FDA 21 CFR 820.30 medical device design and development plan
In the United States, medical device design and development planning has historically been governed by FDA 21 CFR 820.30, which specifies design controls as part of the Quality System (QS) regulation.
Meeting regulatory requirements for medical device design requires manufacturers to establish and maintain plans that describe design and development activities, define responsibilities, and capture inputs from across functions.
Bear in mind, though, that this framework is changing soon. In 2024, the FDA issued a final rule to align more closely with ISO 13485, formally establishing the Quality Management System Regulation (QMSR).
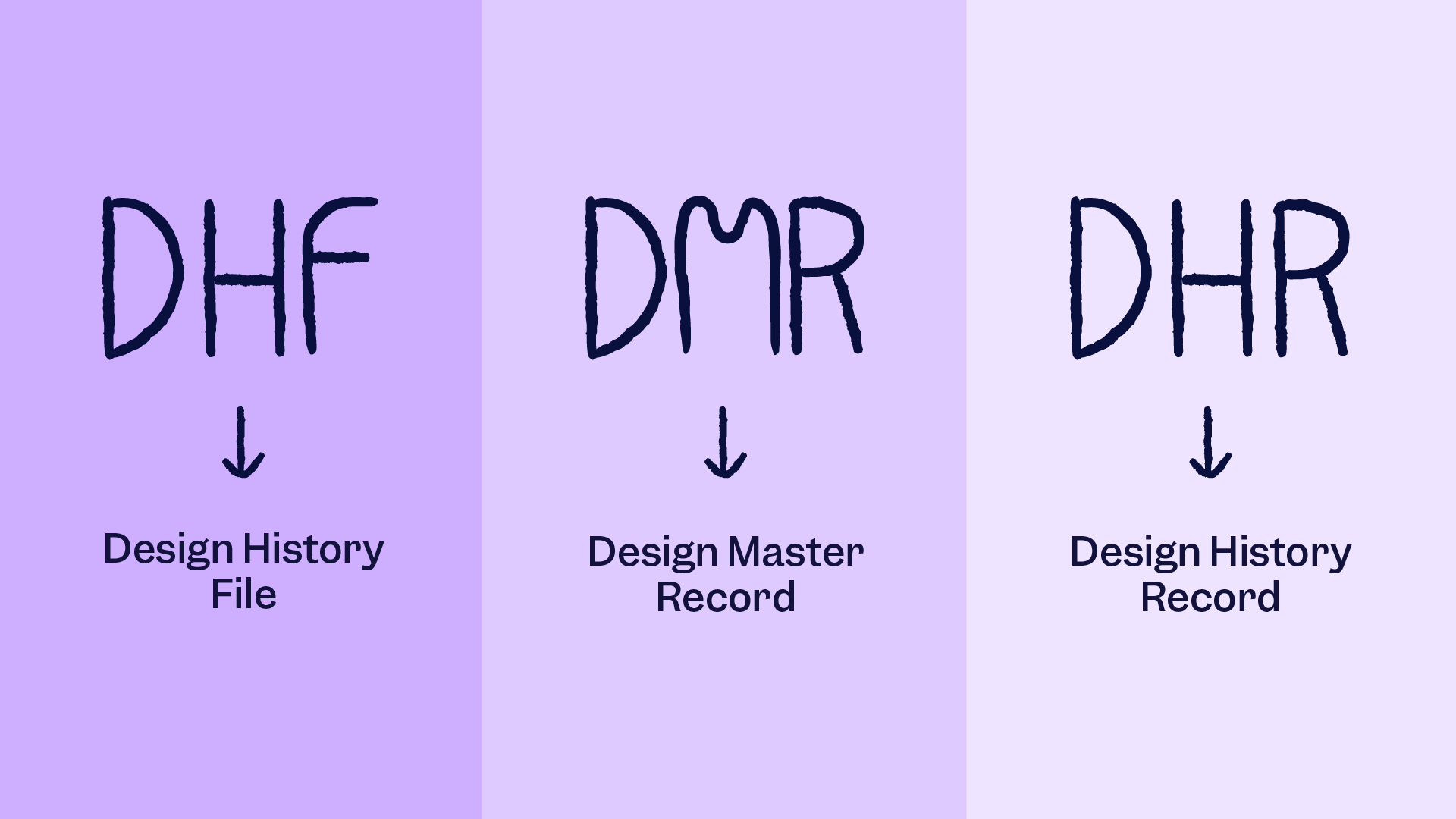
The new requirements take effect in February 2026, modernizing the U.S. approach and promoting global consistency. While harmonizing with ISO 13485 is the primary driver behind QMSR, the FDA has introduced clarifications to preserve critical expectations, including design files, device records, and alignment with the Federal Food, Drug, and Cosmetic Act.
For QA and RA managers, project managers, and regulatory teams, this convergence means design and development plans will need to satisfy both ISO and FDA requirements, but with reduced duplication.
In my opinion, it’s a positive outcome: a clearer, globally aligned standard that supports faster, safer access to high-quality devices. And it means that if you already have an ISO 13485 design and development plan, you’re already well on your way!
Core elements of a medical device design and development plan
Both ISO 13485 and FDA 21 CFR 820.30 require a structured approach to the design and development of a medical device, and this is recorded in a design and development plan.
Here are the core elements you need to include:
- A plan for the entire design and development process.
- Design inputs, and how “user needs” and other design concepts were captured in a way that is clearly defined and measurable, so that the device can be verified and validated.
- Design process where a product is designed to meet the design inputs.
- Design outputs, which include the preliminary and subsequent device designs, as well as prototypes, and evolving versions of the medical device being developed.
- Design reviews are conducted formally and documented. They test the medical devices produced to ensure they are suitable and identify any issues or problems that need to be addressed.
- Design verification is documented evidence that the device has been examined and that the design outputs fulfill the requirements outlined in the design inputs.
- Design validation also involves examination of the device, along with objective evidence that shows the user needs and requirements for the device set out in the design inputs have been met, and the device is fit for its intended use.
- Design changes must be documented, including all changes to the design of the medical device.
- Design transfer involves the details of the processes for transitioning the medical device from design stages to manufacturing, ensuring the device is produced to a consistent quality and meets the correct specifications.

Design and development plan medical device example
Let’s take a look at how an insulin pump would be created according to a medical device design and development plan.
First, the product requirements would be defined. This would include the needs of Type 1 diabetic patients who would rely on the pump for all of their insulin delivery, and Type 2 diabetics who may struggle to meet their glycemic target, as well as technical specifications like size, weight, and power consumption targets.
The product would also need to have a set of core features that users could use to help them choose between the new device and those already on the market.
Next, the insulin pump would need to be designed, taking into consideration what hardware to use, which could involve the development of a new type of microfluidic delivery system, how the device would be powered with maximum battery efficiency, and fail-safe designs to ensure patient safety.
Software would also need to be developed along with the appropriate software, making the insulin pump easy for patients to monitor, for example, on their smartphone.
Importantly, the pump would need to be carefully tested, verified, and validated to ensure that patient and healthcare provider needs are met, along with safety and technical specifications.
I hope this design and development plan medical device example helps give you a clear picture of what it would look like in practice!
And as a final recap, here’s a checklist of everything that should go into your medical device design and development plan!

Expert tips for your design and development plan
Here are my top tips for developing a medical device design and development plan:
- Build a robust QMS that documents everything clearly and in a structured way that is easy to navigate and supports regulatory compliance. Your QMS should include a system for document control, including version control, review, and approval processes. An electronic QMS can be a lifesaver here when it comes to staying organized and efficient!
- Visualize or map out your design process graphically, which can help make the plan much easier to implement and understand.
- Always remember that at the core of medical device design and development is traceability and control over the process of how devices are developed. Ensure every requirement from user needs to performance and regulatory requirements is traceable to the design outputs and verification/validation activities. Document any changes to the design, including the reason for the change, its impact, and approvals.
- Conduct thorough design reviews at key milestones within the process and document the safety and effectiveness of the design.
- Take a proactive approach to audits by performing regular internal reviews to find and fix problems before a regulatory inspector calls! Use these to assess how well you’re adhering to guidelines, standards, and your own processes.
- Leverage digital tools, including automation. Using the right cloud-based QMS software can really help to create a single source of truth and manage your workflows efficiently.
Conclusion
Building a compliant medical device design and development plan can feel overwhelming—especially with the strict requirements of ISO 13485 and FDA 21 CFR 820.30.
That's precisely where Scilife’s QMS software makes your life easier. Instead of wrestling with scattered files, manual reminders, and endless version control headaches, you can use Scilife to bring everything together in one place.
The platform guides you through each stage—capturing design inputs, risks, outputs, and approvals—while keeping full traceability at your fingertips. It’s like having a compliance safety net built into your process: every review, every sign-off, every update is automatically logged and audit-ready.
In the end, creating a CFR 820 or ISO 13485 design and development plan doesn’t have to be a burden. With Scilife, you can reduce stress before inspections, gain confidence that nothing has been missed, and free up more time to focus on moving your project forward.