If you’ve ever tried to keep track of every design input, output, verification, and validation across countless files and spreadsheets, just to ensure data consistency, you know the feeling.
Tabs everywhere. File names ending in final_v2_reallyfinal.docx.
And when it’s time to prepare reports for internal reviews, suppliers, or regulatory submissions? The scramble begins again — hours lost manually piecing together updates from multiple files to build a complete, compliant picture.
We set out to change that with our Design and Development tool and its traceability matrix.
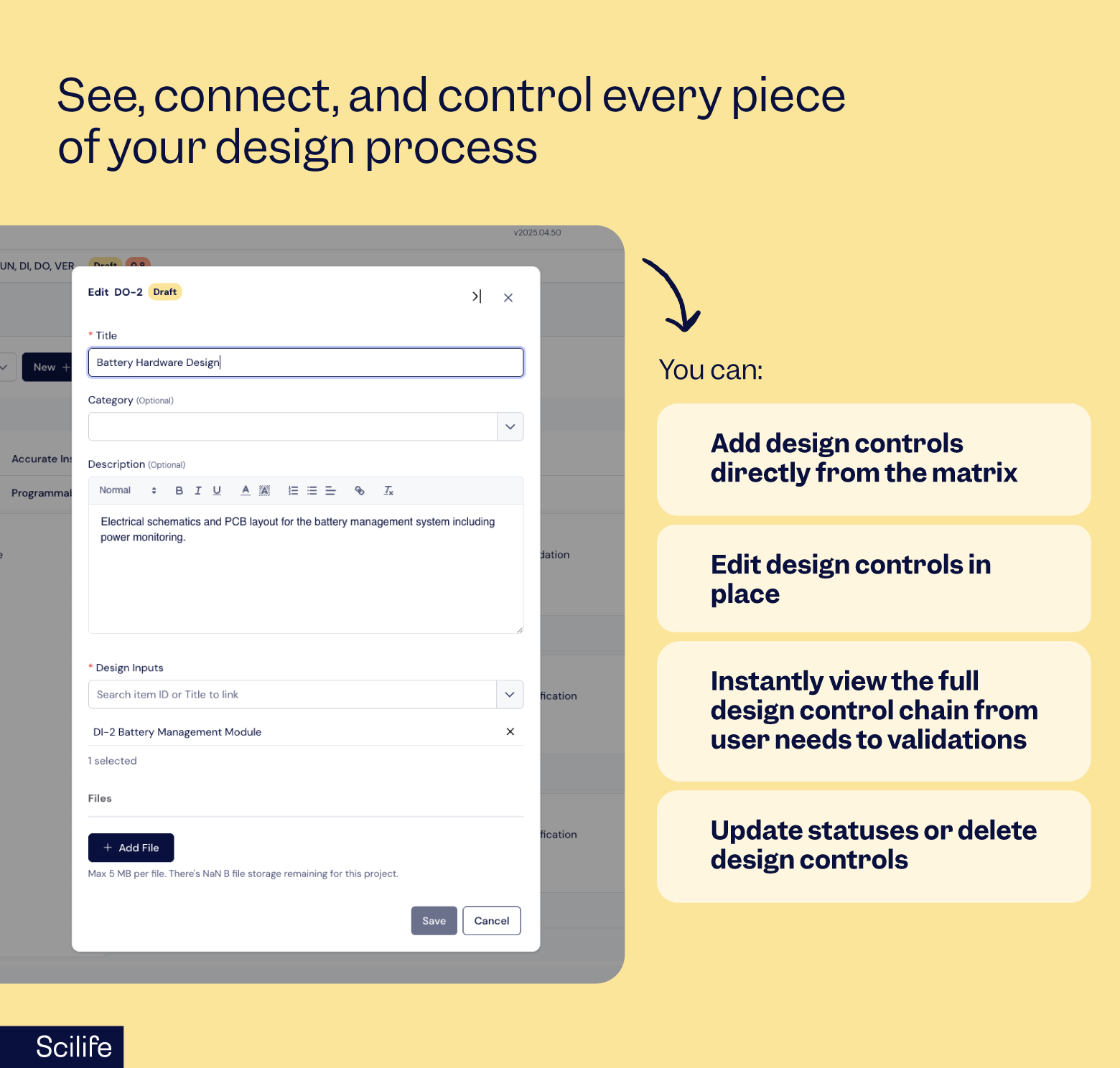
In our latest update, we’ve added something QA and product teams will love — you can now manage the entire traceability of your design controls from a single, intuitive interface and generate complete, audit-ready project reports in just a few clicks.
What’s new in design and development
Interactive traceability matrix
There’s no doubt that maintaining full traceability throughout the design and development of a medical device is one of the hardest tasks for any organization. When done manually, it only increases the risk of human error and consumes valuable resources.
With this update to our traceability matrix, you can now view, add, update, or delete design controls and even manage verification or validation runs — all from a single, intuitive matrix view.
No more hunting through separate files or tabs.
This update turns traceability into a living, breathing part of your daily workflow, not a compliance afterthought.
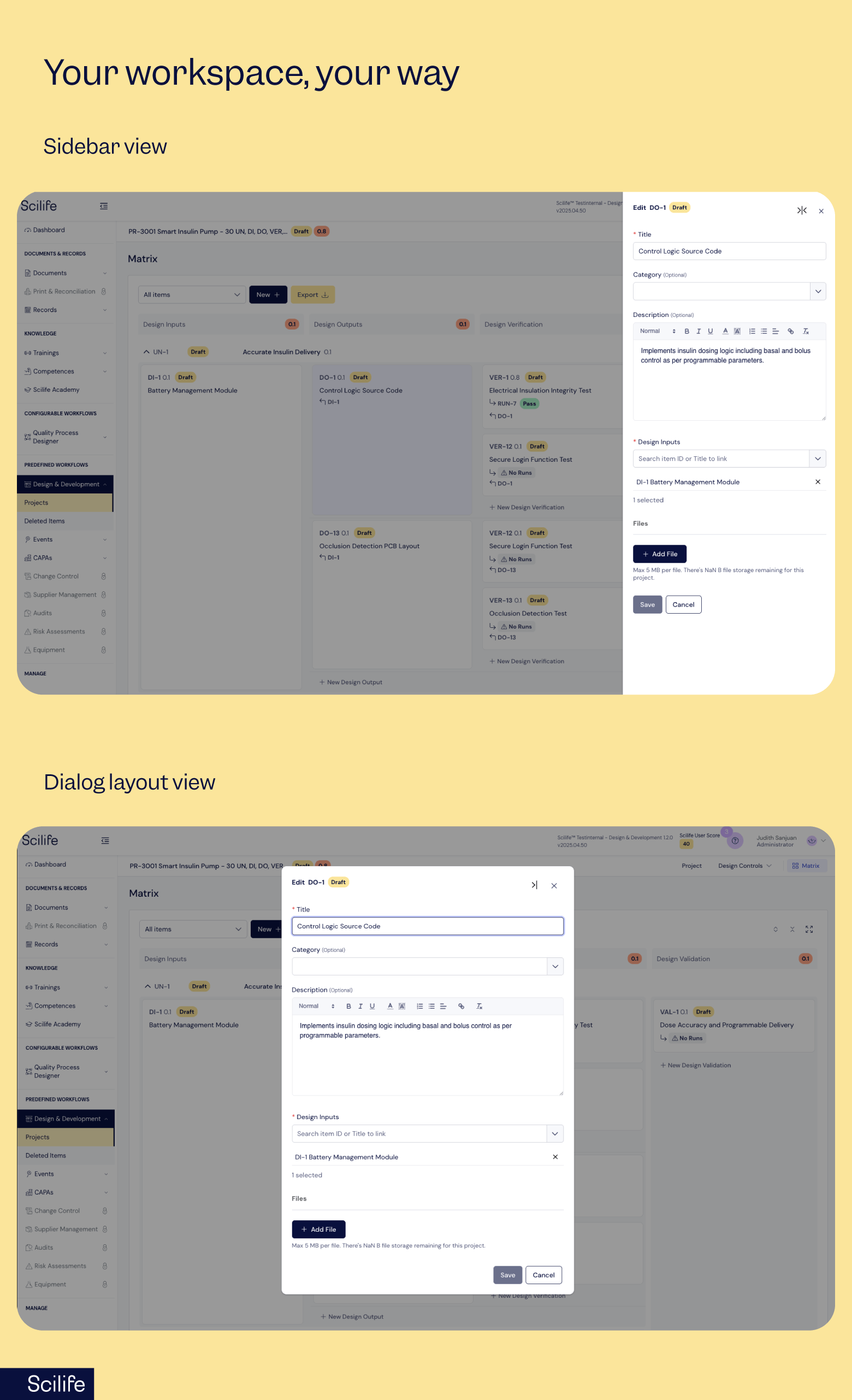
Configurable layouts that match your workflow
Every QA professional works a little differently, and your tools should adapt to you, not the other way around.
Some people like a sidebar view for quick edits while keeping the full project visible. Others prefer a dialog view for focused reviews without distractions.
Now you get to choose.
Switch layouts anytime and work the way that feels most natural to you!
Report Export Wizard
We know reporting can feel like déjà vu, pulling the same data into the same templates over and over again.
And when it’s done manually, it can quickly become a source of risk.
Copying data between files, updating multiple templates, and cross-checking information by hand not only wastes valuable time but also opens the door to inconsistencies and costly mistakes.
That’s why we built the Report Export Wizard, your new favorite shortcut to audit-ready documentation.
- Choose what to include — user needs, design verifications, validations, or all of it
- Pick the format you need (DOCX or PDF) and combine everything into a single file
- Generate, export, and share your report in a few clicks
Your reports are always accurate, always consistent, and always up to date with industry best practice templates in just a few clicks.

Multi-format report export
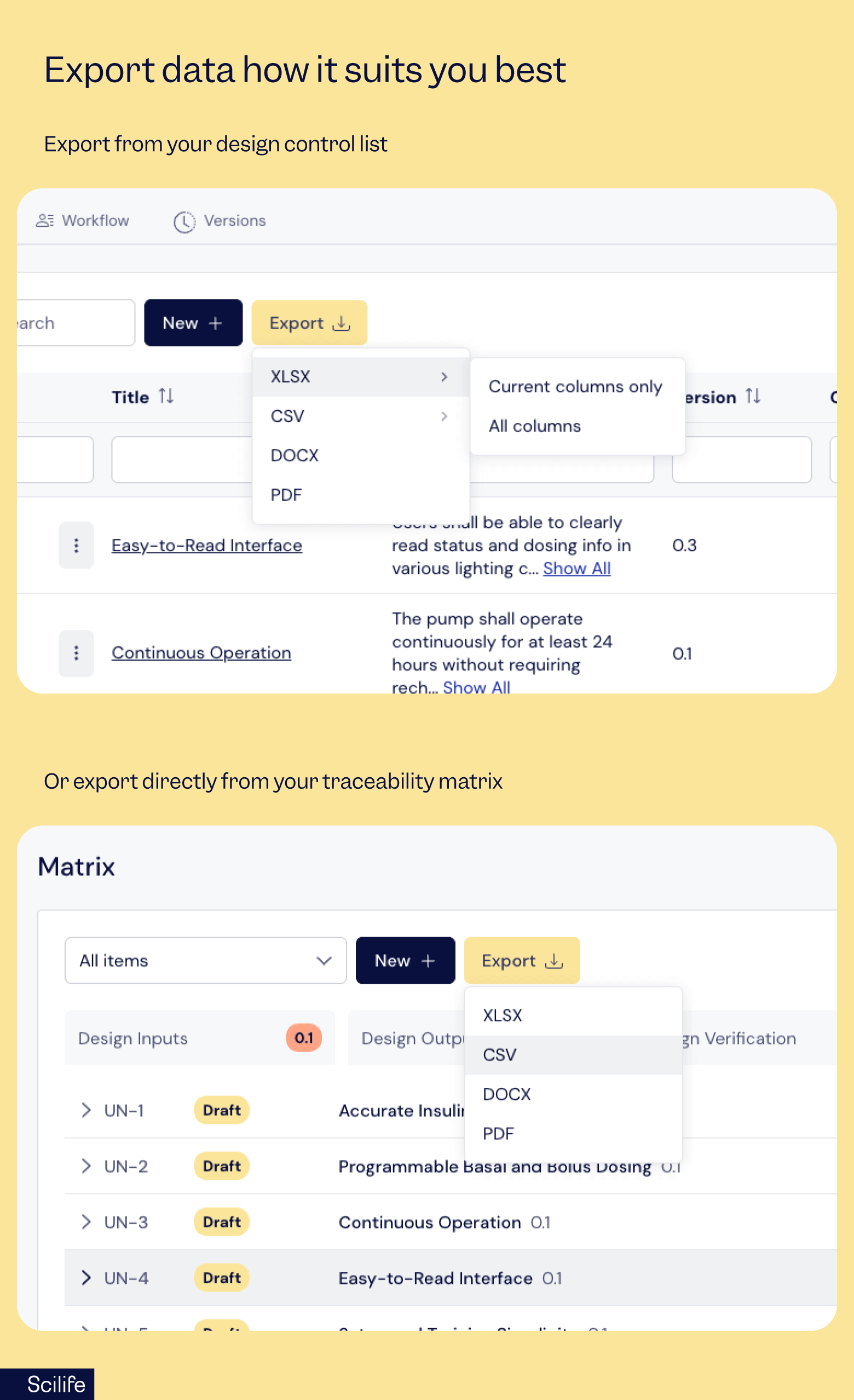
Need more flexibility? You’ve got it!
You can now export data directly from the design control list or traceability matrix in XLSX, CSV, DOCX, or PDF formats.
That means quick analysis for your QA review, polished documents for stakeholders, and submission-ready reports for regulators, all from the same workspace.
A quick recap of why you’ll love this update
Designed for Quality teams who deserve better
We built these updates with one goal in mind: to give quality professionals the control and clarity they need without the chaos.
No more late nights chasing missing links or patching together last-minute documentation.
Now, you can keep everything connected, compliant, and crystal clear, from start to submission.
These new features are live in your Scilife Design and Development workspace.
Jump in, explore the interactive traceability matrix, test the new report exports, and see how much smoother your next audit can be.
And as always, we’d love to hear your thoughts.