Since Brexit, UKCA has replaced CE marking as the route to market in Great Britain. But what that means for medical devices keeps evolving. For manufacturers, quality managers, and regulatory affairs specialists, keeping pace with these changes isn’t just about compliance—it’s about staying competitive in a market where rules are still taking shape.
Whether you’re a UK-based company marketing domestically or an international manufacturer planning to enter the UK, understanding UKCA marking for medical devices is crucial. The timelines, transitional arrangements, and upcoming reforms from the MHRA all impact how you get and keep your device on the market.
In this guide, we’ll explain:
- What is UKCA marking?
- Clarify the latest rules for medical devices in the UK (2025 and beyond)
- Give our expert recommendations on how to navigate the regulatory chaos in the UK.
We’ll also share insights from across the industry on how to stay future-proof as the UK’s “future medical devices regime” comes into force.
Key takeaways on UKCA marking medical devices
What is UKCA marking for medical devices?
So, what is UKCA marking? UKCA (UK Conformity Assessed) marking is the United Kingdom’s own product conformity mark, introduced after Brexit to replace the CE mark for goods placed on the market in Great Britain (England, Wales, and Scotland).
While the idea of UKCA marking was straightforward - “the UK’s version of CE” - the implementation has been more complex. The government introduced multiple transitional timelines to ease the shift, particularly for the medical device industry, where conformity assessments are intricate and resource-intensive.
It’s also worth noting that Northern Ireland follows different rules under the Northern Ireland Protocol. CE-marked devices, and in some cases the UKNI mark, remain valid there - so the UKCA mark alone does not grant market access in Northern Ireland.
UKCA vs CE marking
Now that you know what is UKCA marking, the second most common question for many manufacturers is: “Do I still need UKCA marking if I already have CE marking?”
The short answer is: yes, if you want to sell your device in Great Britain.
The UKCA mark is required for new devices, while CE-marked devices can continue to be accepted under certain transitional rules, currently until June 2028–2030, depending on the device type and certification pathway.
Here’s how the two compare UKCA vs CE marking:
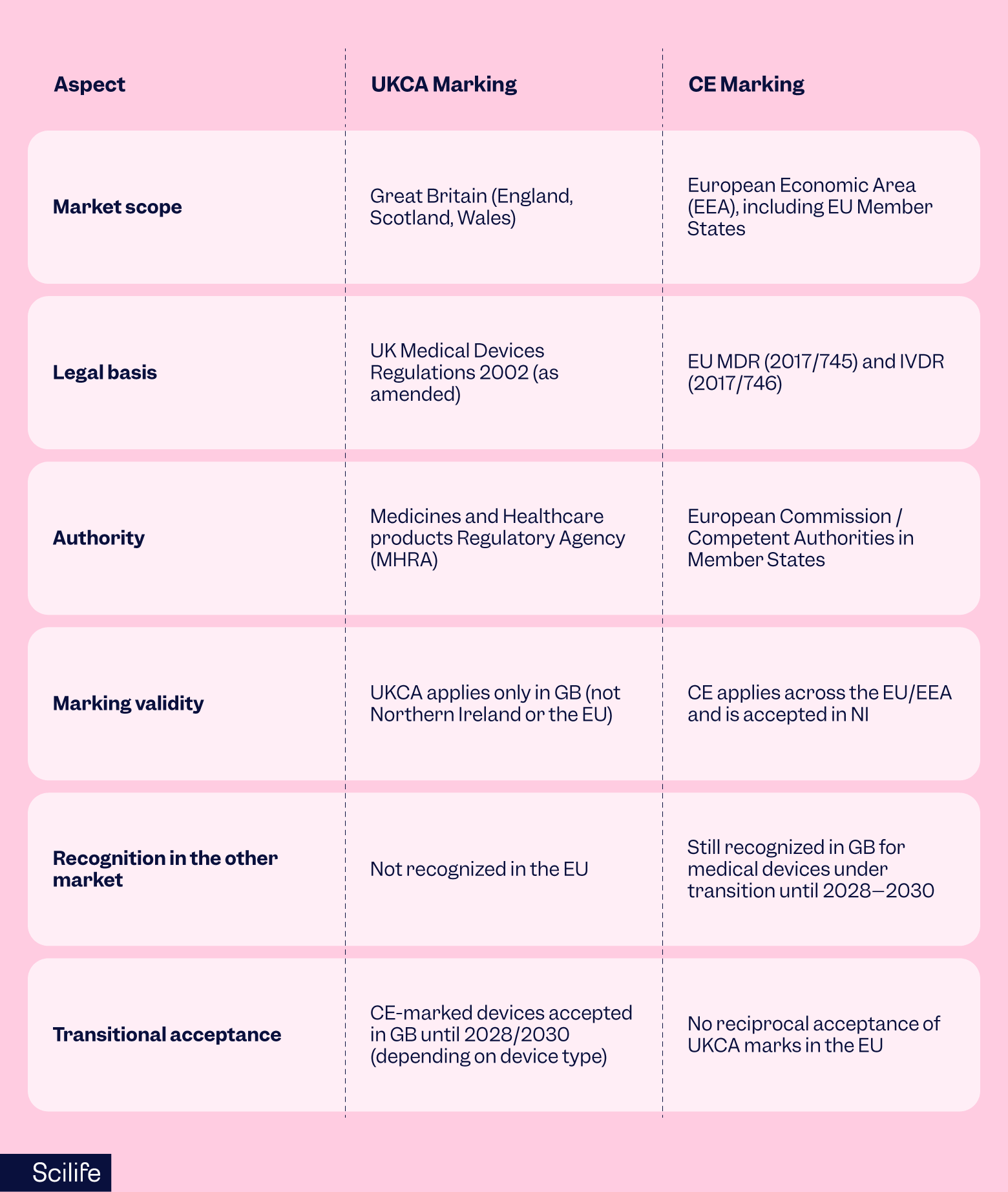
The similarities in process and documentation make dual compliance possible. Many manufacturers choose to dual-mark their products with both UKCA vs CE marking during the transition period.
However, over time, the UK’s regulatory pathway is expected to diverge further as the MHRA introduces its “future medical devices regime,” which came into effect on 16 June.
UKCA marking medical devices: The current regulatory state (2025 and beyond)
So, what does the regulatory environment actually look like in the UK right now? Let’s take a look!
How UKCA and CE coexist right now
Right now, the UK takes a practical, transitional approach: CE-marked devices can continue to be sold in Great Britain, while manufacturers can also choose the UKCA route.
- UKCA route: Devices are assessed under UK law (UK MDR 2002) with conformity checks by a UK Approved Body and MHRA registration.
- CE route (transitional): CE-marked devices remain valid until the certificate expires or the transitional period ends (sometimes as late as 2030), helping prevent sudden supply gaps.
There’s no need to re-certify CE devices immediately - UKCA processes only apply if you switch routes or make major changes.
Recommended learning:
CE approval for medical devices: Steps to market access in the EU
The most important recent MHRA updates on the UKCA marking medical devices (and what they mean)
In July 2025, the MHRA published its response to the consultation on routes to market and IVDs and set out a package of policy changes and next steps. The key industry-impacting shifts are:
- International reliance routes: faster market access for devices already approved by trusted overseas regulators.
- CE mark recognition: a planned consultation on allowing CE-marked devices to stay on the GB market indefinitely.
- UKCA marking: the physical logo may no longer be required once a national UDI system is in place.
- Digital focus: greater use of UDI and registries to reduce paperwork and speed access to safe devices.
These developments show a clear policy direction: faster access via reliance, fewer physical labelling burdens via UDI, and possible indefinite recognition of CE. Most of these are policy shifts or consultations that still need implementation through secondary legislation or further MHRA rules.
UKCA mark: What applies now vs what’s on the horizon
Be careful to separate the operational reality from proposals:
Applies now
- CE-marked devices may continue to be placed on the GB market under transitional rules (subject to certificate expiry and the device/dossier specifics).
- Registration with MHRA is required for placing devices on the market.
- UKCA remains a valid and active conformity route.
On the horizon (policy/consultation stage)
- Indefinite recognition of CE marks (MHRA plans to consult; not yet final).
- Broad implementation of international reliance routes (some routes confirmed; details and eligibility to follow).
- Removal of the physical UKCA logo requirement after UDI is operational (dependent on UDI rollout).
In short, nothing is being forced on manufacturers overnight, but the likely direction is toward reduced duplication and more digital traceability - so planning for both continued CE acceptance and digital/UDI maturity is prudent.
Great Britain vs Northern Ireland: a short clarification on the UKCA mark
- Great Britain: UKCA (or transitional CE) governs market access, with devices registered with MHRA and assessed by UK Approved Bodies.
- Northern Ireland: Follows EU rules under the Northern Ireland Protocol. CE (and where required, UKNI) marking is needed - UKCA alone is not enough. Manufacturers covering the whole UK must manage different labelling and conformity routes.

If CE marking stays, is UKCA still required?
Another common question many manufacturers have been asking recently is, “If CE marking stays, is UKCA still required?
Even if CE marking is recognized indefinitely in Great Britain, UKCA still matters for several reasons:
- Regulatory divergence: The UK may change rules over time, making CE alone insufficient for new devices or major updates.
- Local obligations: GB requires MHRA registration, UK-specific post-market reporting, and a UK Responsible Person for non-UK manufacturers.
- Operational simplicity: UK-only companies may prefer UKCA to avoid EU paperwork and dual-QMS overhead.
So, who should pursue UKCA?
- UK-based manufacturers for simpler MHRA compliance.
- Manufacturers planning major or frequent device changes.
- Manufacturers seeking faster GB market access or using UK Approved Bodies.
- Organizations wanting resilience via dual-marking.
Scilife Tip:
Even if CE recognition becomes indefinite, UKCA can still act as useful insurance. If the cost is reasonable compared with the risk of future rule changes or disruption, it’s worth getting UKCA certification now.
For EU-only sellers, UKCA is less urgent but worth considering for future strategy.
If your GB exposure is low, keep an eye on things and make sure registration, UKRP, and post-market obligations are covered. Early adopters often gain extra confidence, but each company should choose the path that fits its product risk, markets, and pace of change.
How to stay future-proof in the UK market as a medical device company
Regulatory uncertainty doesn’t have to stall your UK strategy. Whether CE recognition becomes indefinite or not, the smartest move for most medical device companies right now is dual readiness - keeping both CE and UKCA pathways live so you can pivot quickly as the rules evolve.
Let’s look at how to make it manageable in practice.
- Maintain parallel documentation: share overlapping technical files, but keep UKCA-specific annexes (MHRA registration, UK Responsible Person, UKCA labeling) and track certificates separately.
- Use a cloud-based QMS: manage updates, version control, and audits efficiently without doubling workload.
- Engage Approved Bodies early: clarify classification, get feedback, and leverage modular assessments for faster submissions.
If your GB market share is small, or you rely heavily on CE, you might instead:
- Maintain CE as your base route, but register with MHRA and monitor the consultation on indefinite recognition.
- Use international reliance routes if your device is already cleared in the US, Canada, or Australia - this may offer faster GB access than UKCA.
- Prepare for UDI readiness - even if UKCA labelling becomes optional, traceability and post-market monitoring will remain key.
Conclusion: The future of UKCA
Companies that act early on UKCA and/or dual-marking gain a real competitive advantage: smoother audits, faster adaptation to future rules, and stronger confidence from partners and regulators alike.
UKCA is still evolving, and so should your quality system. Continuous learning and digital readiness will help you stay flexible no matter how the MHRA’s roadmap unfolds.
FAQs
Is UKCA still required?
Is the UKCA mark replacing CE?
Is CE marking still valid in the UK?
Yes, under transitional rules:
General medical devices until certificate expiry or 30 June 2028.
IVDs until certificate expiry or 30 June 2030.
Do I need both CE and UKCA marking?
It depends:
- Selling only in Great Britain via UKCA? UKCA alone is enough.
- Selling in both the UK and EU? Dual marking is recommended.
- Already CE-marked and selling in GB? You may not need to switch immediately but should plan ahead.
Can I use the same technical file for CE and UKCA?
Yes, most of the documentation overlaps. You’ll need to add UK-specific elements like:
- Compliance with UK MDR 2002
- UK Approved Body certificates (if applicable)
- UK Responsible Person info, MHRA registration, and local post-market requirements