Quality control (QC) processes can be a significant bottleneck in the smooth functioning of a pharmaceutical manufacturing unit if a lot of material gets stacked into either incoming raw material or the quarantined area before it gets approved. The challenges arise as manufacturers must ensure that all the material they purchase from the suppliers meets the internal specification criteria. Therefore, all incoming raw materials and in-process materials are tested inside the QC facility before they are used in the next stage of manufacturing the finished products. Similarly, the finished product must be tested in the QC facility before it is delivered to the market.
Because of varying market needs, various amounts of material are stacked for testing. Therefore, most QC labs handle a tremendous amount of work pressure throughout the year. The procedures and devices used for testing different raw materials, in-process materials, and finished products are different, which adds complexity to the day-to-day tasks performed in QC labs. If a material is not tested, then it is piled up in the designated quarantine areas before it is being used.
Suppose large amounts of materials are piled up in the storage area of the manufacturing facility. In that case, it also affects the manufacturing of the next batch owing to a lack of storage space. That is why debottlenecking the QC processes is essential for the smooth functioning of the Pharmaceutical manufacturing unit.
Let’s discuss various approaches to debottlenecking QC procedures in the pharmaceutical industry to embrace the best practices for the future.
Implementing Operational Excellence And Lean Thinking
Like all other processes in the manufacturing unit, operational excellence (often referred to as OPEX), and lean thinking can significantly simplify the workflow of QC processes. Let’s take a look at the most important standard methodologies in lean thinking that can add value to your QC processes.
Value Stream Mapping of Quality Control Procedures
Value stream mapping is a lean thinking methodology in which each process step is documented as a flowchart. Then, each step is evaluated for its value addition to the entire process. Typically, every step is either classified as a value-adding or non-value-adding step. Any value-adding step is an essential step without which it would be impossible to have a tangible end product. A non-value-adding step is a step that leads to lean waste such as excess motion, production, processing, wait time, transportation, defects, and inventory. Generally, a non-value-adding step can be performed more effectively with alternative steps or can be completely eliminated from the workflow.
For example, suppose the manual sign-up process for approving raw and in-process materials and finished products in the QC process results in a long wait time, excess inventory, and excess motion of employees. In that case, the organization can decide to replace it with digital signatures using a Smart Quality eQMS. After the organization implements the change, the manual sign-up process step in the workflow will be eliminated from the flowchart and replaced by the digital sign-up process using the Smart Quality QMS.
Standard Work in Quality Control Procedures
As the name suggests, standard work is all about building uniform systems across the organization so that there is unambiguity regarding micro-details of a given task, including the basics of when, who, where, and how the task will be performed. This helps avoid so-called eleventh-hour confusion among employees: Everybody knows what is expected from them, when, where, and how they should take action.
In an organization that uses Smart Quality tools for managing the QC processes in the organization, the performance metrics of the employees take into account their key roles and responsibilities concerning Quality. Hence, all standard work is implemented by default as employees are already fully aware of their responsibilities based on the key performance metrics and can access necessary work instructions, standard operating procedures, and relevant processes within the Smart Quality management software itself.
Drum Buffer Rope in Quality Control Procedures
Drum Buffer Rope (DBR) applies the theory of constraints to manufacturing operations. Implementing DBR smoothens the flow of constrained operations owing to a capacity shortage or restrained resources. The process of implementing the DBR involves identifying the least efficient process and then working on the elimination of the constraint by efficient management of resources.
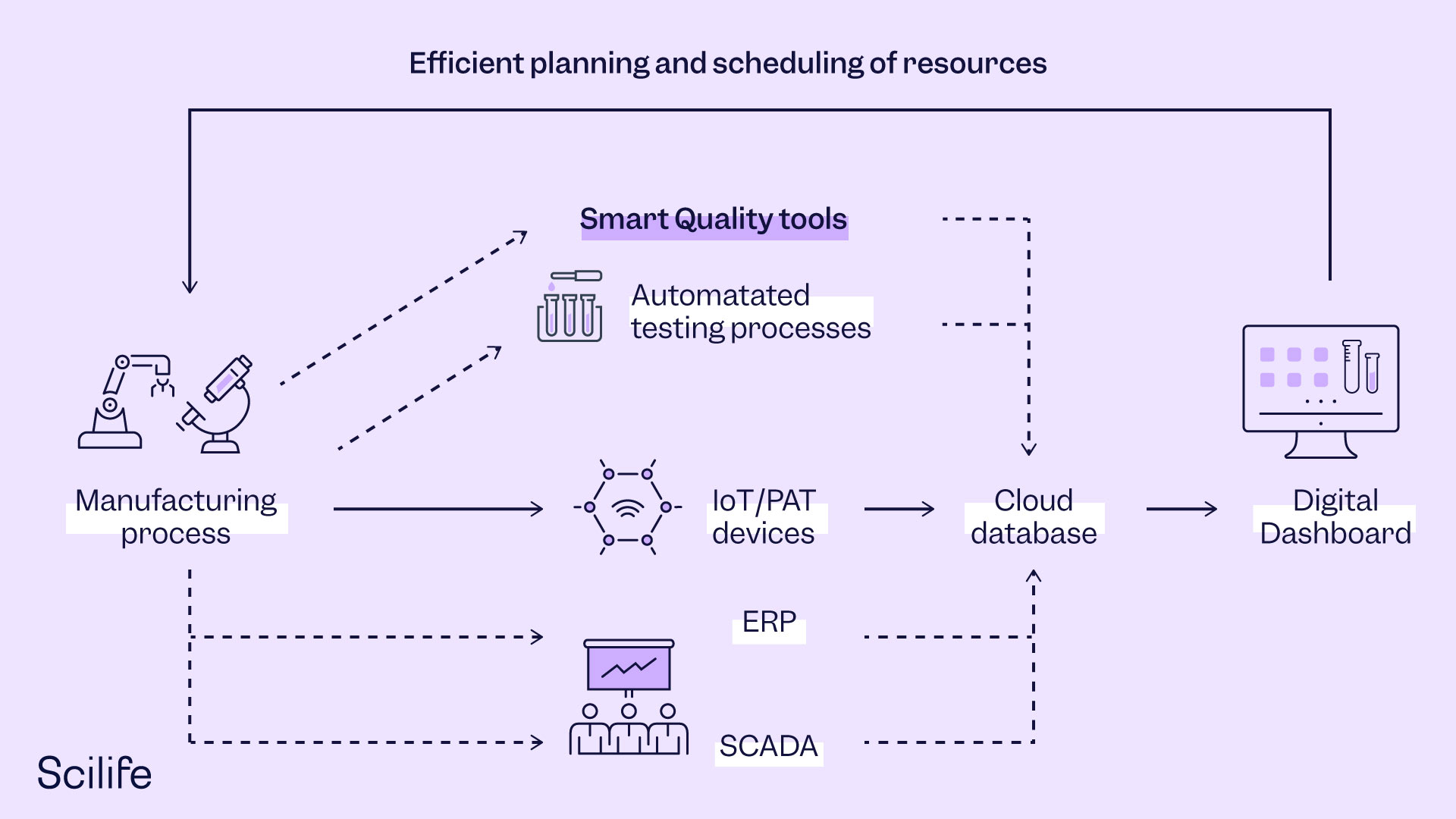
Pharmaceutical organizations can implement DBR in QC procedures using digitally enabled Smart Quality management software with a dashboard facility. The dashboard gives a quick overview of the Quality metrics for all products and processes in the manufacturing unit. It can be used to identify in-efficient processes that have poor Quality metrics. Poor Quality metrics owing to repeated deviations and CAPAs cause a delay in rejecting or accepting the batch. Sometimes the manufacturer may decide to rework a batch, which again results in more samples to be tested in the QC lab. However, organizations can use DBR to focus their resources on eliminating repeated deviations and CAPAs by performing a root cause analysis. In this way, if the constraints in inefficient processes are managed promptly, then it results in the smooth execution of manufacturing workflow without burdening the QC staff.
Another feature that will drastically improve dashboard functionality is the integration of the dashboard with QC instruments, resource availability, and LIMS (Laboratory Information Management Systems). The integration will empower managers to plan more efficiently and schedule QC lab activities dynamically by merely glancing at the dashboard.
Automation in QC Procedures
One often-quoted golden rule in QC is to “never automate tasks that can be eliminated, and never delegate tasks that can be automated or streamlined.” Therefore, before opting for automation, you should always ensure that the selected procedure for automation cannot be eliminated or streamlined using the value stream mapping concept found in lean thinking.
Sampling Automation in QC
There are several essential tasks in the QC laboratory that can be automated. For example, the use of robotics for sample injection and sample preparation is very commonly automated in most QC laboratories in the 21st century. Additionally, processes like sample withdrawal and collection can also be automated to save QC staff time and effort.
Process Analytical Technology in Quality Control
The complete end-to-end automation of the QC process is also possible if we take the analytical instrument to the manufacturing process rather than taking samples from the manufacturing process to the QC laboratory. This can be achieved using the Process Analytical Technology (PAT) tools mentioned in the FDA’s PAT guideline.
PAT simplifies QC procedures to a great extent as it doesn’t require sample preparation thanks to non-destructive testing capabilities. PAT implementation has more advantages if the PAT tool is in-line with the manufacturing process flow compared to off-line or at-line placed tools. As in-line PAT tools provide instant real-time insights, they represent the entire batch population. Near-infrared spectroscopy and Raman spectroscopy are the most widely used PAT sensors in the pharmaceutical industry at this time. Scientists have started to use data generated by these sensors to build chemometric models for the prediction of critical Quality attributes of pharma products. In addition to spectroscopy, process data from SCADA can be used to build multivariate chemometric models that predict Quality attributes in real-time.
The Future of Quality Control Procedures
Technology will simplify future QC procedures like never before. The QC personnel will no longer have to run here and there. Technology will simplify the management of QC activities through IoT database integration. Digital dashboards will display decisive information by collecting cloud-based data from various interfaces such as e-QMS, PAT/IoT tools, SCADA, and ERP. These dashboards will play an instrumental role in dynamic scheduling and inventory management. Digitalization will enable managers to manage resources more efficiently using updated tools and methods like digital Kanban. Digitalization will also help companies to go paperless for Quality management, and advanced analytics will be used to monitor Quality in real-time. Automated sample preparation, collection, and testing will simplify QC further. Various digital sensors and process/product modeling will be used to monitor Quality accurately and without delay. Advanced technologies, automation, and data-driven decision-making will reduce laborious activities to a great extent. All of these new opportunities will enable QC experts to think more critically and successfully about building intelligent automated systems.
Conclusion
QC procedures can be a bottleneck in meeting market needs if planned inefficiently. Manufacturers can overcome the hurdles in inefficient planning by embracing various operation excellence practices, lean thinking, and Smart Quality principles for smoother functioning of QC procedures. The future QC procedures will leverage digitalization, Smart Quality tools, live status dashboards, and real-time QC through process automation and modeling. Many leading players in the pharmaceutical industry are already in the process of implementing future Quality procedures. If your organization is not working on this already today, then it’s time to gear up for the ongoing change as soon as you can.
Discover how a Smart Quality QMS for Pharma can help you improve your processes with Advanced Analytics!