Quality Control
Quality control (QC) is designed to maintain or improve the quality of a product.
Of course, there’s a lot more to the process than that. Read this glossary term to learn about all things QC.
What Is Quality Control?
Quality control plays an essential role in the Life Sciences space. The process requires teams to build an environment where members can work toward manufacturing perfection—ensuring the product the company is building meets customer, stakeholder, and regulator standards.
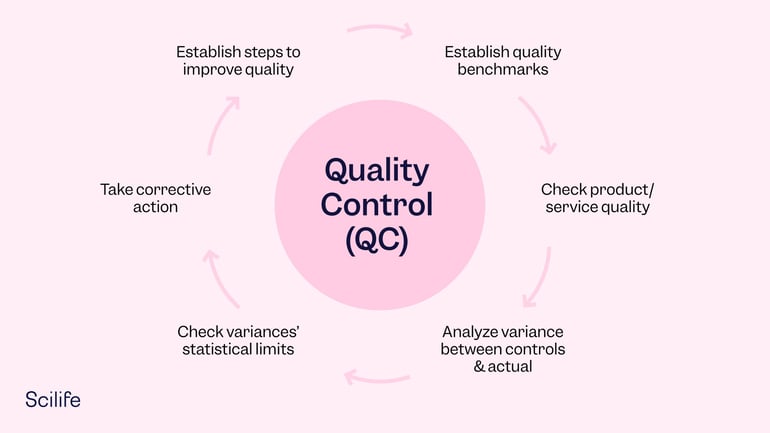
At the core of this process is the establishment of well-defined controls. The controls are meant to standardize the manufacturing process. This means that if the product attributes don’t align with the controls, then corrective action must be taken. In this way, QC can elevate production and ensure quick and consistent responses to quality problems.
The idea behind QC is to reduce or eliminate errors by making sure the right people are performing the right processes at the right time when manufacturing a product. Together, teams can establish benchmarks for product quality and test products to eliminate variations from the controls mentioned above.
By eliminating variations, quality leaders can rest assured that all the units going to market will resemble the final product. They can achieve this by testing raw materials, taking samples, and remediating problems or discrepancies when needed. This is where a QC inspection may come in. We’ll touch on this process in more detail in the next section.
Why Is Quality Control Important?
Quality control promotes not only the quality of a given product, but overall company success. It also helps manufacturers elevate their reputation and stay compliant with regulators.
ISO 9000—the set of quality management standards with which most Life Sciences companies are very familiar—defines quality control as “part of quality management focused on fulfilling quality requirements.”
This is rooted in three aspects including:
- Controls, defined process, performance criteria, and the identification of records.
- Competence including knowledge, qualifications, skills, and experience.
- Qualitative elements such as integrity, company culture, confidence, and quality relationships.
These aren’t the only components that make up quality control. Inspection plays an important role as well. Inspection may involve the visual examination of a product, the chemical or microbiological testing of a product, or even the analysis of a service. Inspectors can check for defects or variations in any number of ways, depending on the industry and the scope of the QC inspection.
Should the inspector identify a problem, they can resolve the issue themselves, send the product back to the manufacturer for remediation, or reject the product in its current form. Inspectors are tasked with notifying their supervisor when they come across an issue. That way all parties can work together to address the discrepancy and eliminate quality problems.
In short, quality control keeps defective products from going to market.
Quality Control vs. Quality Assurance
QC and QA (quality assurance) share some similarities. However, they are two different concepts.
While quality assurance focuses on how a product is made, quality control focuses on quality management. Quality management includes the activities involved in maintaining a standard of excellence in the manufacturing process.
Think of it this way: QC is product-oriented and aims to identify defects, while QA is process-oriented and aims to prevent defects.
How to Measure QC Performance
There are a number of ways quality leaders can evaluate the performance of their QC strategy.
Here are just some of the ways quality leaders can evaluate their QC performance:
Taguchi Method
This QC method focuses on research and development, as well as product design and development. The overarching goal is to lower the probability of product defects or failure. The Taguchi Method considers the design process more important than the manufacturing process. It’s ideal for those set on eliminating production defects before they occur.
X-Bar Chart Method
This QC method will reveal whether the manufacturer’s products and processes meet the required specifications. The chart is designed to determine whether defects occur at random or systemically. The process involves selecting product units at random, and then testing them for one or more attributes. The Y axis tracks variances in the product, while the X axis tracks the actual product samples.
There are two different types of charts you might see here.
A chart that analyzes a single product attribute is known as a univariate chart.
Meanwhile, a chart that measures variances across numerous product attributes is called a multivariate chart.
100% Inspection Method
This QC method evaluates all the different aspects of a product in a single inspection. The main focus here is to rule out defects or flaws that will impact the product quality. This method is so specific that it may require manufacturing data and software. The benefit is that it’s very thorough, but one drawback is that looking at the product in its entirety can (occasionally) cause further defects during the inspection.
The way companies approach QC—and the evaluation of QC performance—will depend on their organizational goals. There are a range of process improvement techniques available today, so you’ll have plenty to choose from. Chances are you’re already familiar with Six Sigma and Lean Six Sigma, for example.
Conclusion
All organizations can benefit from a comprehensive quality control process, and Life Sciences companies are no exception. Maintaining quality control not only protects product quality, but it also enhances organizations’ overall success. QC often makes teams feel happier in the workplace and more satisfied with the company culture. It can even give team members a sense of ownership over the products they help to create. The benefits of quality control are truly far-reaching, spanning from company leaders and stakeholders, to team members and the customers themselves. Perhaps most importantly, QC increases safety for everyone involved.
Do you want to read more about the differences between QA and QC?
Check out this article.
Additional resources

How to Implement the Continuous Improvement Cycle | Scilife
Even an organization with stellar leadership and a solid core of employees experiences hiccups from time to time. Despite having assembled all the ...

How to assess and enhance your Quality Management Maturity | Scilife
As the life sciences industry becomes increasingly regulated and competitive, quality management has become more vital than ever. Are you confident ...

Best Quality Management Software (QMS) for Life Sciences | Scilife
The right electronic Quality Management System (eQMS) can help strengthen your compliance processes and build a culture of quality within your ...

How to write a good quality plan for medical devices | Scilife
In life sciences, especially if you’re in the medical device industry it becomes harder to manage projects in accordance with your company’s quality ...
Turn quality into your brightest asset with Scilife
