If you’ve been on the lookout for a go-to resource offering valuable tips for technical writing, your search is finally over. We’re here to simplify complexity.
In highly regulated industries such as the life sciences, technical communication isn’t something you can simply pick up on the job; it requires deliberate practice and knowing where to start. Our comprehensive guide aims to do exactly that – help build your foundations in effective technical writing.
Whether you’re a beginner technical writer, a subject matter expert, a technical documentation manager, or a project manager, we’re here to increase your knowledge and skills to take your technical writing to the next level. Soon, you’ll be confidently writing a wide range of technical documentation including manuals, procedures, work instructions, deviations, root-cause analysis and CAPAs.
For visual learners, we have a specialized video designed to help you effortlessly improve your technical writing skills below.
Understanding the basics: expert tips for technical writing success
Before exploring best practices and tips for technical writing, let’s start with the fundamental question: what is technical writing?
Technical writing is a writing discipline that is often defined as the art of “simplifying the complex”.
Documents such as procedures and work instructions contain complex information that needs to be communicated in a simple way. They must be useful, understandable, and accurate for audiences that need to use the information to perform activities that meet a specific business objective. Thus, the primary goal of technical writing is to enable readers, regardless of their prior knowledge, to comprehend and apply the information effectively.
Technical writing includes creating documents for both print and digital formats. Making sure these documents are well-planned and well-written is crucial in the life sciences for any process or product.
Here are some common terms that you should be familiar with:
Technical document
A technical document is a written material with a technical focus, helping individuals (or multiple individuals) to execute a process or procedure, or use a product. Examples include user guides, SOPs, manuals, work instructions, quick reference guides, and similar materials.
Technical writer
A technical writer is a writer who develops (e.g., writes, edits, or curates) technical documents.
Subject matter expert
A subject matter expert is a person who is an expert on a particular topic.
Writing involves more than just style, grammar, punctuation, and editing - this constitutes only a small portion, about one-fifth, of the entire process. The broader aspects of planning, structuring, reviewing, and publishing are often overlooked.
Why is technical writing important?
Navigating technical communication can seem straightforward, but it's easy to inadvertently make it more complex than necessary.
Life sciences is an industry where we can see the direct impact of written workplace communication. Clear technical communication can make all the difference between a simple work instruction and multiple standard operating procedures (SOPs). It also plays a crucial role in ensuring tasks are executed accurately on the first attempt, preventing deviations caused by misunderstandings.
How to write technical documents effectively?
Here are some best practices and tips for technical writing to keep in mind when creating documents in a regulated environment:
- Be clear and precise: Strive for clear and precise language to convey information accurately and comprehensively.
- Define the audience: Identify both primary and secondary readers, ensuring your document addresses their specific needs and levels of expertise.
- Be objective: Maintain objectivity by steering clear of emotional or subjective content. Rely on facts and figures without making assumptions or inviting subjective commentary.
- Be consistent with grammar and style: Maintain a uniform approach to grammar and style in your document to improve both its readability and overall professionalism.
- Do not use vague language: Use precise language to provide clarity and eliminate ambiguity. Avoid vague terms such as ‘soon’, ‘later’, ‘as soon as possible’, ‘often’, ‘sometimes’, ‘frequently’, ‘some’, ‘quite’, ‘many’, ‘a few’, etc.
- Opt for the active voice: Use the active voice over the passive voice whenever possible to foster directness and accountability in your writing.
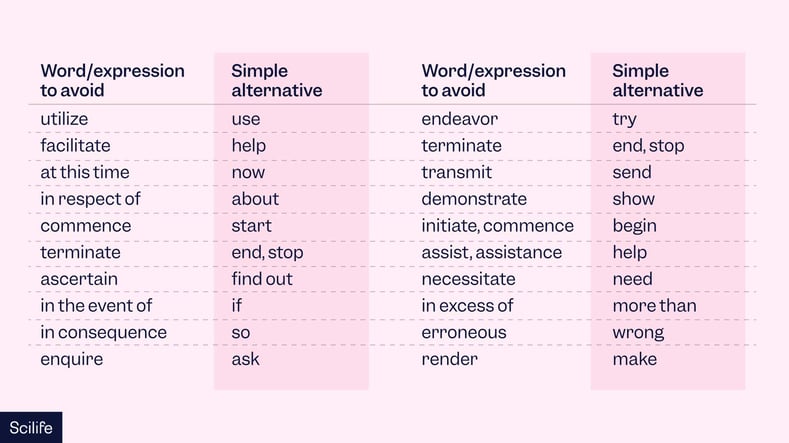
- Use simple words and expressions: Aim for accessibility to a broad audience by ensuring your language is straightforward and easily understandable. Avoid jargon.
Sentence and paragraph length in technical documents
Contrary to what you may think, it’s not smart to write long, complicated sentences in the life sciences. Effective technical writing is all about keeping sentences short and simple. Consider these tips for technical writing:
- Each sentence should share just one piece of information. Avoid using compound sentences if you can.
- Long paragraphs are not good, just like long sentences.
- A paragraph should focus on one clear idea. Keep all paragraphs shorter than half a page.
- However, if your paragraphs are too short, it might be hard to read. This happens when your thoughts are not well-organized. If you have a series of sentences with different ideas, it's usually better to use bullet points or lists.
Good technical writing examples of the active voice
In technical writing, the vast majority of sentences should be written in the active voice. It is more concise, direct, and easier for readers to understand.
In the active voice, an actor acts on a target.
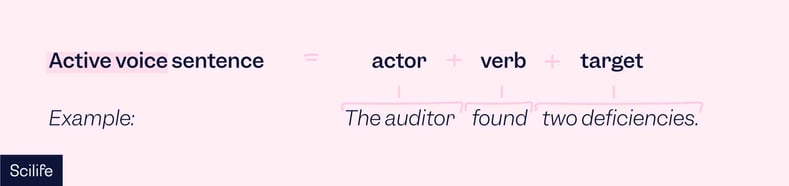
For example: “The auditor found two deficiencies.” In this case, “the auditor” is the actor and appears at the beginning of the sentence.
By contrast, the sentence in the passive voice reverses the formula.
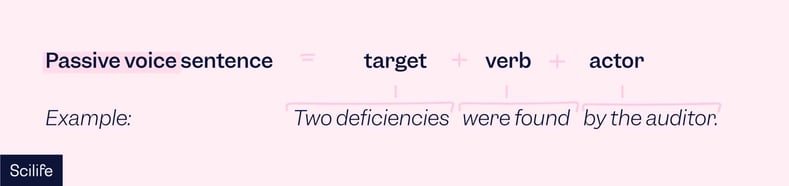
For example: “Two deficiencies were found by the auditor.” Here, “the auditor” is the actor, but it appears at the end of the sentence.
The active voice is used when you need to provide clear, direct instructions, or when you need to describe steps in a procedure or process like in SOPs, work instructions, guides, and so on.
Here are some more good technical writing examples that demonstrate the difference between the active and passive voice.
Active: "The software identified the error."
Passive: "The error was identified by the software."
Active: "The project manager will make the decision."
Passive: "A decision will be made by the project manager."
Active: "The user submitted a request for information."
Passive: "A request for information was submitted by the user."
Active: "The quality assurance team reviewed the report."
Passive: "The report was reviewed by the quality assurance team."
Technical writing tips for deviations, root-cause analysis and CAPAs
When writing deviations, root-cause analysis reports, and CAPAs, there are some key tips for technical writing that you should follow.
Remember to always be clear and specific, offering exact and precise data. Your document should eliminate any doubts or room for interpretation. This ensures that your information is detailed and accurate, setting a foundation for effective communication.
Also, don’t forget about the Five Ws (and one H): What, Who, When, Where, Why, and How. They will help your writing be clear and helpful for understanding and solving problems.
Discover the best approach for various types of technical documentation below.
How to document deviations
When writing deviations, stick to presenting facts, data, or observations before, during, and/or after the event. Organize all details chronologically, including names of individuals, equipment IDs, instruments, date, time, and other relevant information. Describe the measures taken to control or limit the event. However, remember to keep it concise—avoid unnecessary details and repetition. Focus on explaining the facts without delving into the "why."
Explain the requirements or specifications affected by the deviations. Clearly reference the applicable guidelines, regulations, or policies, providing clause numbers and paragraphs for precise documentation.
How to write a root-cause analysis report
Root-cause analysis is a systematic method for investigating the fundamental cause of an event or deviation. Various tools, such as the 5 Whys process or the Ishikawa diagram, can be employed by organizations for this purpose. Regardless of the tool used, the investigation should comprehensively document all findings based on evidence.
In cases where the investigation is related to an out-of-specification result in quality control, a thorough examination should be conducted to identify potential analytical errors. This necessitates a comprehensive review of the analytical process, including sampling, reagents, instruments, and procedures.
Historical data should be trended and analyzed, with the results summarized in tables and graphs. It is vital to report affected materials and batches promptly as a deviation may impact product quality or other related products. Immediate attention should be given to addressing potential risks. Besides reporting affected lots, it is advisable to include any other lots that could have been affected.
In instances where the root cause is not identified, it is imperative to propose the most probable root cause based on a scientific hypothesis.
How to write CAPAs
It's crucial to acknowledge that root-cause analysis might occasionally misidentify human error or equipment failure as the primary issue. While these factors may play a role, effective CAPA actions must delve deeper to address the actual root cause.
For instance, simply conducting a training session may not always be the exclusive or most effective CAPA action. Errors can stem from poorly described procedures. Therefore, the CAPA action should concentrate on enhancing the procedure or modifying the process to ensure a more comprehensive and lasting solution.
| Document | Purpose | Key components |
| Deviation | To document and analyze events or occurrences that deviate from standard processes or specifications. |
|
| Root cause analysis | To systematically investigate and identify the fundamental cause of an event or deviation. |
|
| CAPA |
To address and rectify the root cause of an issue identified through root-cause analysis, ensuring it does not recur. |
|
Technical writing checklist for SOPs and Work Instructions
The majority of the technical writing tips that we have already shared will apply to the development of SOPs and Work Instructions. Here’s a quick reminder:
- Clarity is key: Clearly and concisely present each idea.
- Active voice, present tense: Prefer active voice and use present-tense verbs for clarity and immediacy.
- Simplify language: Use straightforward language to enhance understanding.
- Employ action verbs: Ensure instructions are definitive, leaving no room for ambiguity.
- Concise sentences and paragraphs: Keep sentences and paragraphs short for readability.
- Step-by-step format: Present information in a clear and easy-to-read format.
Here are some additional considerations that will prove invaluable when creating SOPs and Work Instructions:
- Clearly define scope: Specify what, where, and when the procedure applies.
- Establish responsibilities: Clearly outline the roles and responsibilities involved.
- Outline the purpose: Articulate the purpose explicitly, especially for work instructions.
- Tailor to the audience: Consider the end-user; your writing will differ based on the audience, whether it's an operator, analyst, manager, auditor, regulatory body, etc.
- Process description: Use steps and stages to delineate a process effectively.
- Lists and bullet points: Utilize lists and bullet points for clarity and emphasis.
- Incorporate visuals: Enhance comprehension with tables, diagrams, pictures, etc., when applicable.
- Abbreviations list: Include a list of abbreviations for quick reference.
- Use print screens: For IT procedures, integrate print screens for visual guidance.
- Reference other documents: Include references and links for more detailed information.
Key takeaways
Effective technical writing is a tough skill to master and like all skills, demands plenty of practice. Although well-written technical content is often rare, a few simple changes can transform an average piece into a great one. By implementing our tips for technical writing, you can avoid common mistakes such as overcomplicating content or being long-winded. Your documentation will not only meet necessary standards but will also serve as a reliable and insightful resource for thorough analysis and resolution at your organization.