Change is essential for growth, no matter which sector your company or organization excels in. However, change can also be a trying period in any company unless you and your team are well prepared for it. The best way to prepare yourself and your team for the inevitable change is to implement a strong change management process within your company.
If you are looking forward to implementing a robust change management process in your company too, then read the article to discover 7 steps you and your team should consider thoroughly before embarking upon the change journey.
1. Identify Your Reason for Change
There could be several reasons for implementing a new change in your company. Being aware of why you want to implement change in your organization will help you to manage it effectively. Therefore, identifying the reason for the change is the first step in the transition process. For example, the possible reason for a change may be because you want to offer new services or products. Another reason might be that you'd like to streamline your operation to make it more convenient for your customers, or you're using older technology and want to transition to newer software and hardware. It could also be that a crucial new regulation or ISO requirement is imminent, and you need to change your organizational processes to comply with the new regulation. Whatever the reason, make sure you clearly identify it before implementing the change.
In addition to identifying the reason for the change, the implementers should also start organization-wide awareness campaigns to educate employees about the importance of implementing the change. According to experts in the field, great implementers spread the word about change in advance to create an atmosphere of inclusiveness and enthusiasm across the organization. However, while creating the buzz, it is essential to recognize that few employees are interested in their employer's share price or return on equity. Therefore, leaders should methodically cascade a compelling change story through the entire business rather than spamming everyone with generic communications materials. It's a tricky balance: the core message must be equally purposeful for the entire workforce, yet it also must be personal and relevant to the specific audience.
2. Take Note of Any Industry Regulations
Once your entire workforce is aware of the importance of the change, it is time to understand how you can implement the change while complying with applicable legislation. Regulated industries like pharma, biotech, and medical devices require additional research and planning regarding organizational change. You have to consider multiple legalities and take note of any special rules or regulations that govern what you can and cannot do. You want to keep these on hand throughout the transition to ensure that your organization doesn't experience any legal setbacks. The purpose of the regulation is to ensure that changes made to a product or manufacturing process do not compromise the safety or efficacy of the product.
For example, if you need to make post-approval changes in a drug substance manufactured by your company, then you must duly notify the FDA of any changes made to a product or manufacturing process. These changes may be classified as either significant or non-significant, with significant changes requiring prior FDA approval.
3. Perform a Risk and Impact Assessment
With the regulations catered for, the next thing to do is ensure that you are changing for the better! You can do this by performing the risks and impact assessment of any type of change that you want to make in your organization. It will help you assess if your changes will improve your processes or products and extend your organization's relevance and longevity. With this assessment, you will know which goals will work best for your organization, and it can get everyone involved in the change management process on the same page. Various risk assessment tools are available that can help significantly with this step.
4. Get a Cost Estimate
Now that you are sure that you are changing for the better, it is time to perform a cost-benefit analysis to understand the relationship between the relative costs and benefits of the proposed change. Therefore, start searching for a cost estimate once you identify your reasons for the change or transition. This will help you get a clear picture of what your organization can and can't afford, and you'll also learn about alternatives to help you achieve your goals.
5. Implement Change Control
Once your organization decides to go ahead with the change and the change control is approved after completing the first four steps (reason, regulations, risk assessment & cost analysis), you should create a comprehensive Change Control Action Plan.
A well-documented Change Control Action plan helps to keep your organization on track throughout the transition. Below are some of the advantages of having a well-documented Change Control Action Plan:

6. Implement your Action Plan
An action plan describes the steps you intend to take before and after the change. In order to provide the conditions for implementation before implementing change, you need to take several actions. Similarly, after the implementation, there are Quality Assurance (QA) and Quality Control (QC) actions that must be taken. Finally, before you're able to implement the change officially, you might need to take one or more of the following actions: validation, verification, qualification (such as Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification PQ)).
7. Set up Quality Assurance
With clear goals outlined, you can start setting up processes to ensure that your organization maintains the same level of quality throughout (and after) the transition period. Ideally, you want to improve your organization's quality assurance, but it's more important to monitor it during the transition period and work to keep it up to your standards. This could include setting goals, identifying your success factors, implementing electronic signatures for your employees, listening to your customers, and taking steps like additional training or correcting your processes to streamline them.
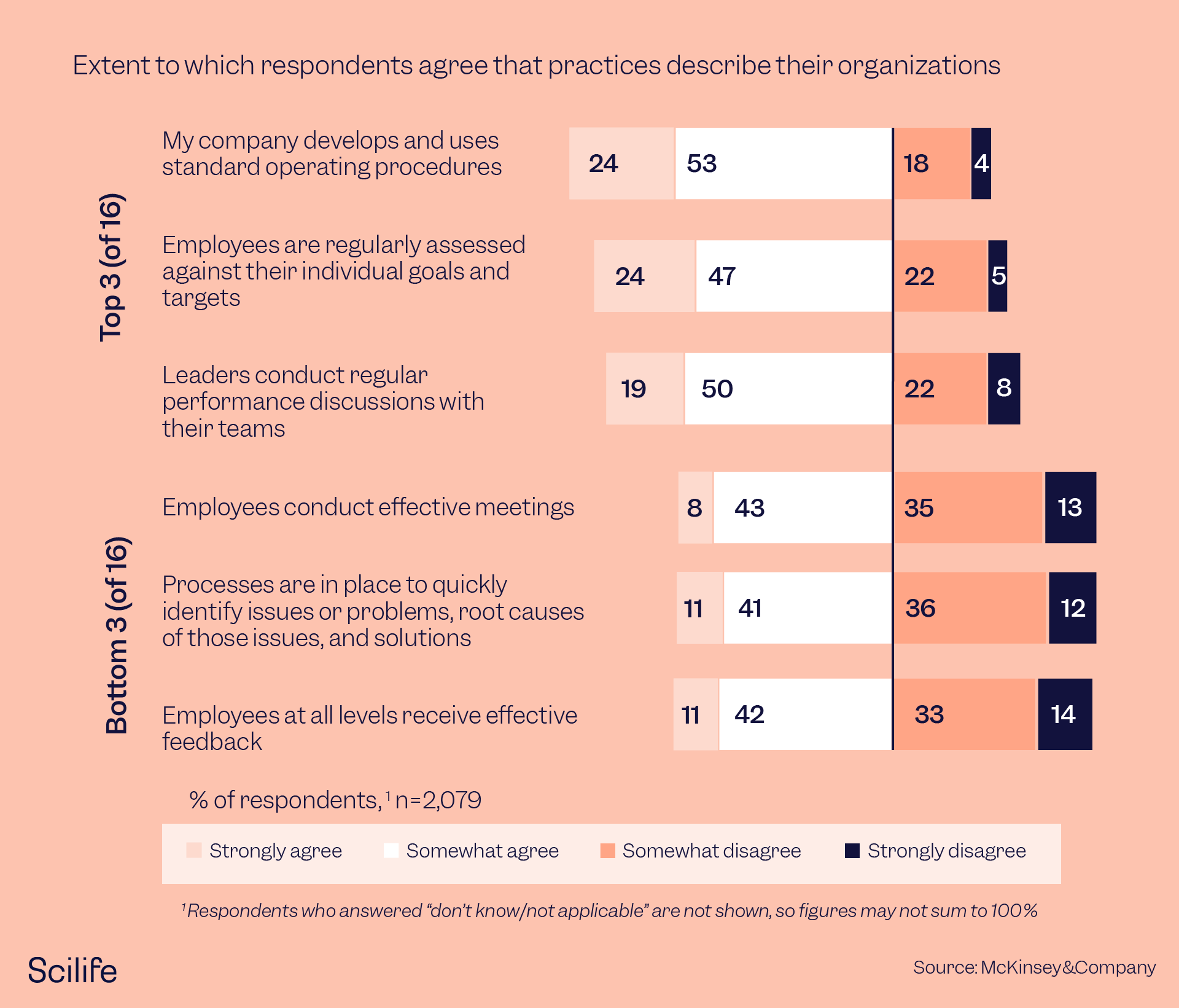
According to a McKinsey survey, the companies that succeed in making changes stick to set-up quality assurance practices such as developing standard operating procedures and periodic employee assessments against their individual goals. The graph below depicts the difference in transformational practices followed by top companies that made changes stick and the bottom companies that failed to make the changes stick in the same survey.
Conclusion
Even a carefully planned change may fail if the change management is inefficient. On the other hand, efficient change management can turn action plans into reality. Companies with efficient change management strategies drive better performance and financial results. Additionally, change management helps companies to anticipate and mitigate probable adverse effects of changes and helps to ensure that changes align with overall organizational goals and objectives.
Make change simple with Scilife
Ready for change, about to start a transition, or already in the middle of it? Our Change Control Software not only helps you monitor and implement a simple and solid quality assurance process for change but also gives you an incredibly powerful tool that can help you maintain that process of change and make small but vital continuous improvements.