EMEA Office
Louizalaan 489
1050 Brussels
Belgium

Quality Assurance (QA) departments usually struggle with engagement from the rest of the company with quality related tasks and processes. Actions are often signed off late, which causes compliance issues. It’s a real headache chasing down employees, which is why an automated system to notify users about tasks and deadlines is so useful.
QA demands continuous visibility into the performance of any quality system. This is necessary to stay compliant at all times and to pass audits with ease. It's not an easy feat to obtain relevant insights through key metrics, such as "average elapsed days per status" for document reviews or "completed late" versus "completed on-time" for training.
Validating a new application to meet compliance regulations during implementation is one thing, but keeping it validated after every upgrade is another (and expensive) story. Unless a Software-as-a-Service (SaaS) solution is prevalidated to meet ever changing regulatory standards, things will get messy, quick.
Finally, a centralized place for all your company’s quality data! Scilife is easily accessible by your whole team from any device, and you regulate who has access to what by setting user rights. Automated notifications and reminders encourage everyone to perform their allocated actions on time, and all users can see their pending tasks on their individual dashboards.
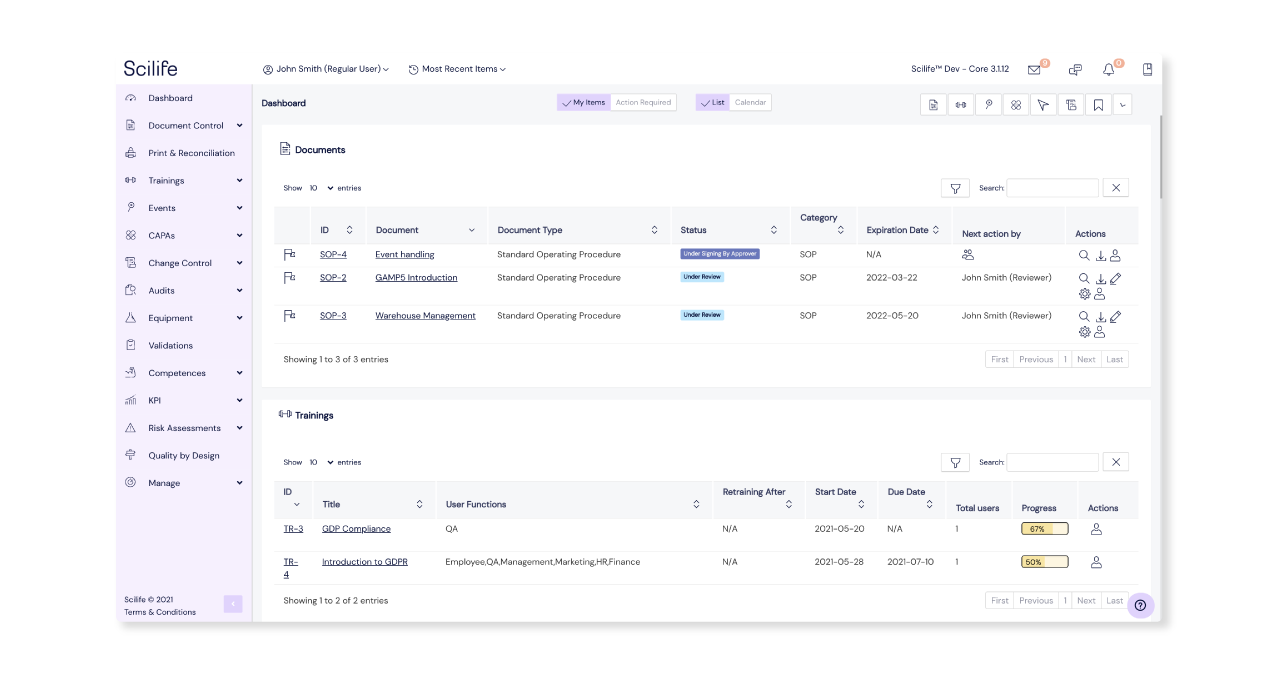
Make complying with regulatory requirements simple and frustration-free. Since all quality data is readily available at any time and is merely a few clicks away, Scilife is an indispensable tool to pass audits quickly and easily.
Scilife comes with a full GAMP5 validation documentation package; drafted, executed and signed off by our in-house regulation experts. This effectively takes away 95% of your validation cost and effort. We make sure you have an updated validation package for every upgrade we release as well, for total peace of mind and inspection readiness.
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
PRODUCT
INDUSTRIES
RESOURCES
COMPANY
Contact Us
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2026 Scilife N.V. All rights reserved.