G2’s Relationship Score identifies software products that provide the best relationship between software sellers and buyers. It’s based on factors including an exceptionally high quality of support, likelihood of recommendation to peers, ease of doing business and more.
Scilife now holds the esteemed title of ‘leader’ on the G2 Grid® in the Pharma & Biotech Software category, a great achievement!
Best Pharma and Biotech Software
OVERALL RANKINGS - G2 SUMMER REPORT 2022
#1 - Most Popular across all segments
#1 - Highest Rated across all segments
#1 - User Satisfaction across all segments
#1- G2 Score across all segments
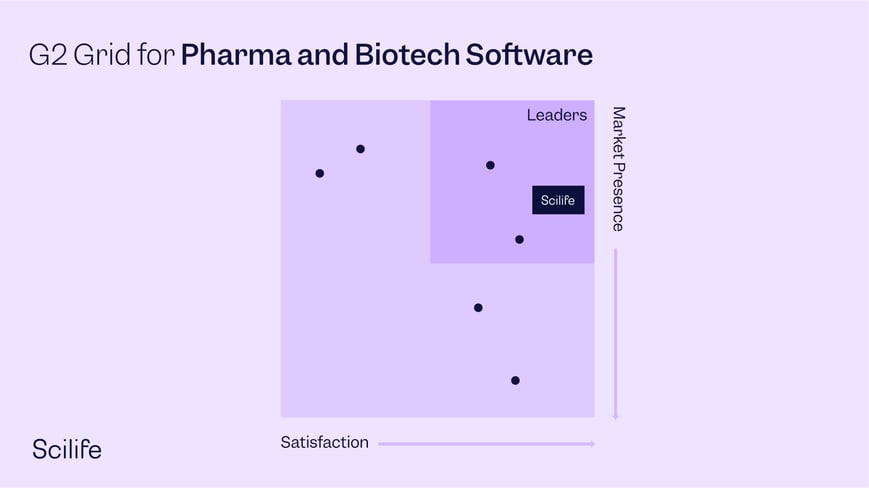
The G2 Grid® is a ranking system that offers a real-time score incorporating both user satisfaction and market presence of the software when compared to similar solutions. G2 takes pride in unbiased reviews and does not allow paid placements in any of their ratings (Source: G2.com, Inc.).
Needless to say, Scilife is thrilled with both the recognition and that their customer-centric efforts are appreciated by their users. The future looks bright for the growing company!

