Scilife has earned two much-coveted High Performer badges in both of the G2 categories in which it’s listed, Best Medical Quality Management Systems (QMS) and Best Pharma and Biotech Software.
Scilife’s aggregate star rating shines at 4.5 stars out of 5 (Source: G2.com, Inc.)
Scilife features on G2.com, the renowned business software review site, in two categories: Medical QMS and Pharma & Biotech Software. The recently released Spring 2022 report places Scilife firmly in the ‘High Performer’ quadrant of their G2 Grid® for both categories. The G2 Grid® is a ranking system that offers a real time score incorporating both user satisfaction and market presence of the software when compared to similar solutions. G2 takes pride in unbiased reviews and does not allow paid placements in any of their ratings.
Here’s how Scilife fared in Q1 2022:
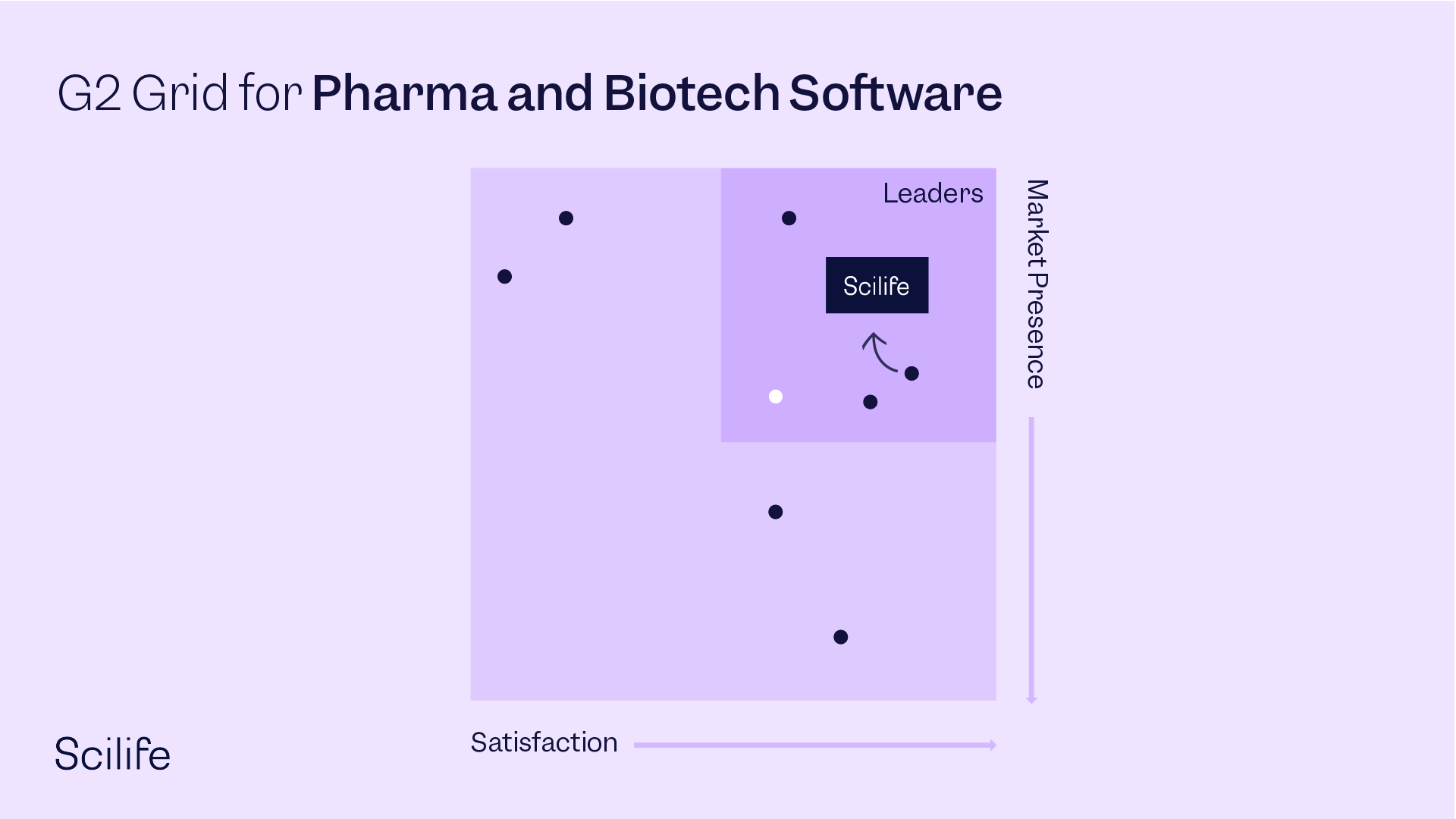
Best Pharma and Biotech Software
OVERALL RANKINGS - G2 SPRING REPORT 2022 - 78 contenders
#1 - Most Popular across all segments
#1 - Highest Rated across all segments
#1 - User Satisfaction across all segments
#2 - Easiest to use across all segments
#2 - G2 Score across all segments
High Performer overall & #2 overall
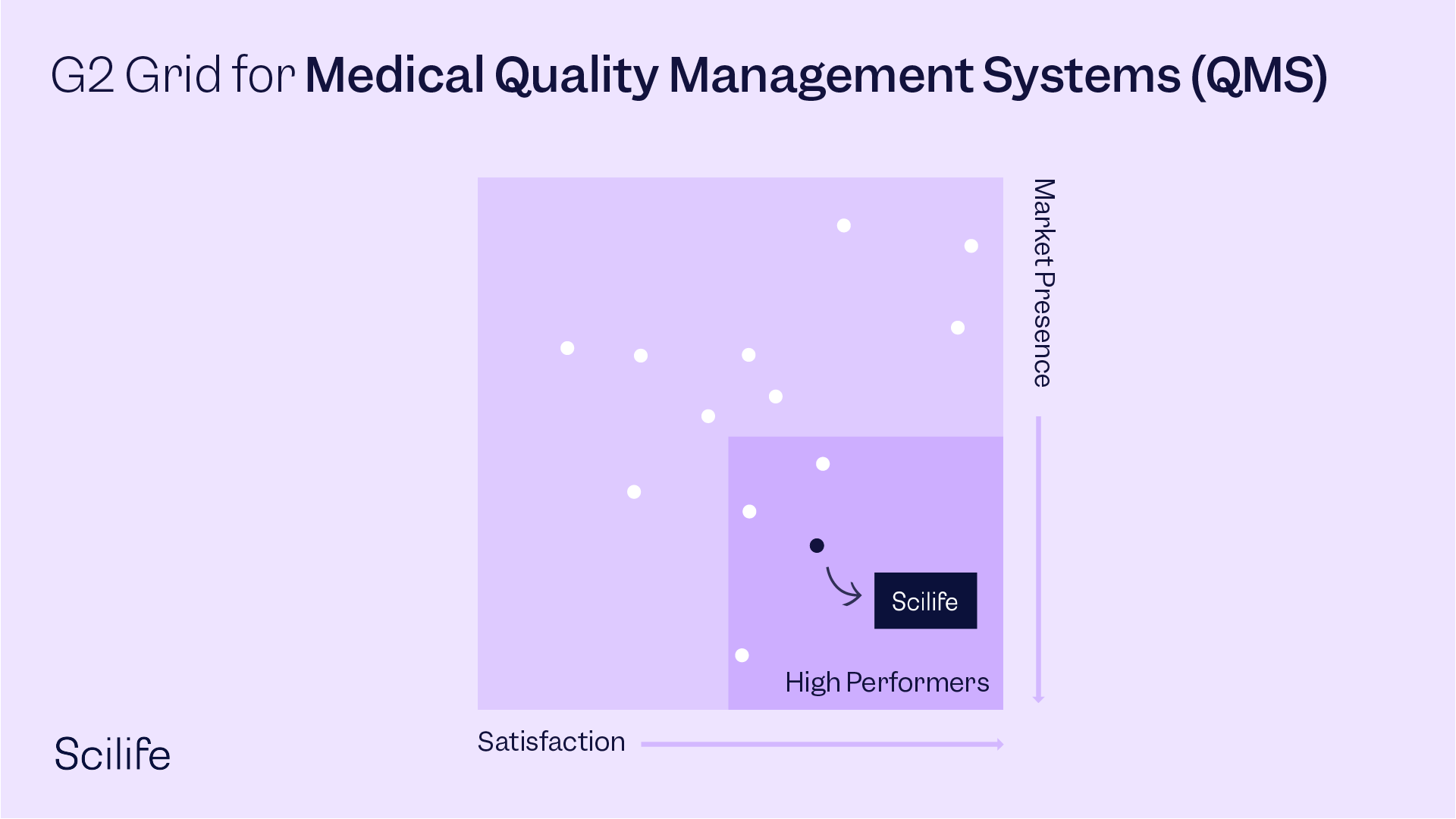
Best Medical Quality Management Systems (QMS)
OVERALL RANKINGS - G2 SPRING REPORT 2022 - 96 contenders
#5 - Highest Rated across all segments
#6 - User Satisfaction across all segments
#11 - Most Popular across all segments
#13 - G2 Score across all segments
High Performer overall
These commendable results show Scilife holds a solid position in the marketplace, standing up on its own even among industry heavyweights. When it comes to Pharma and Biotech, Scilife is unrivalled in popularity and user satisfaction.
Are you a Scilife user?
If you haven’t yet left a review, now is your chance to make yourself heard - you’ll be part of shaping the Scilife platform! What do you think of Scilife and where can the software improve? Your feedback will be an asset to all future users.
Have your say on Scilife’s review page on G2.com
Are you thinking about switching from a paper-based system to eQMS and still not sure what is the best option?
Solve all your doubts by reading more about
the main advantages of a cloud based eQMS.

