EMEA Office
Louizalaan 489
1050 Brussels
Belgium

Meeting Good Manufacturing Practice (GMP) standards is no small feat.
It requires flawless documentation, strict process controls, and effective risk management to ensure product quality, safety, and consistency across every stage of manufacturing.

Our GMP-compliant solution strengthens manufacturing quality and process control from start to finish.
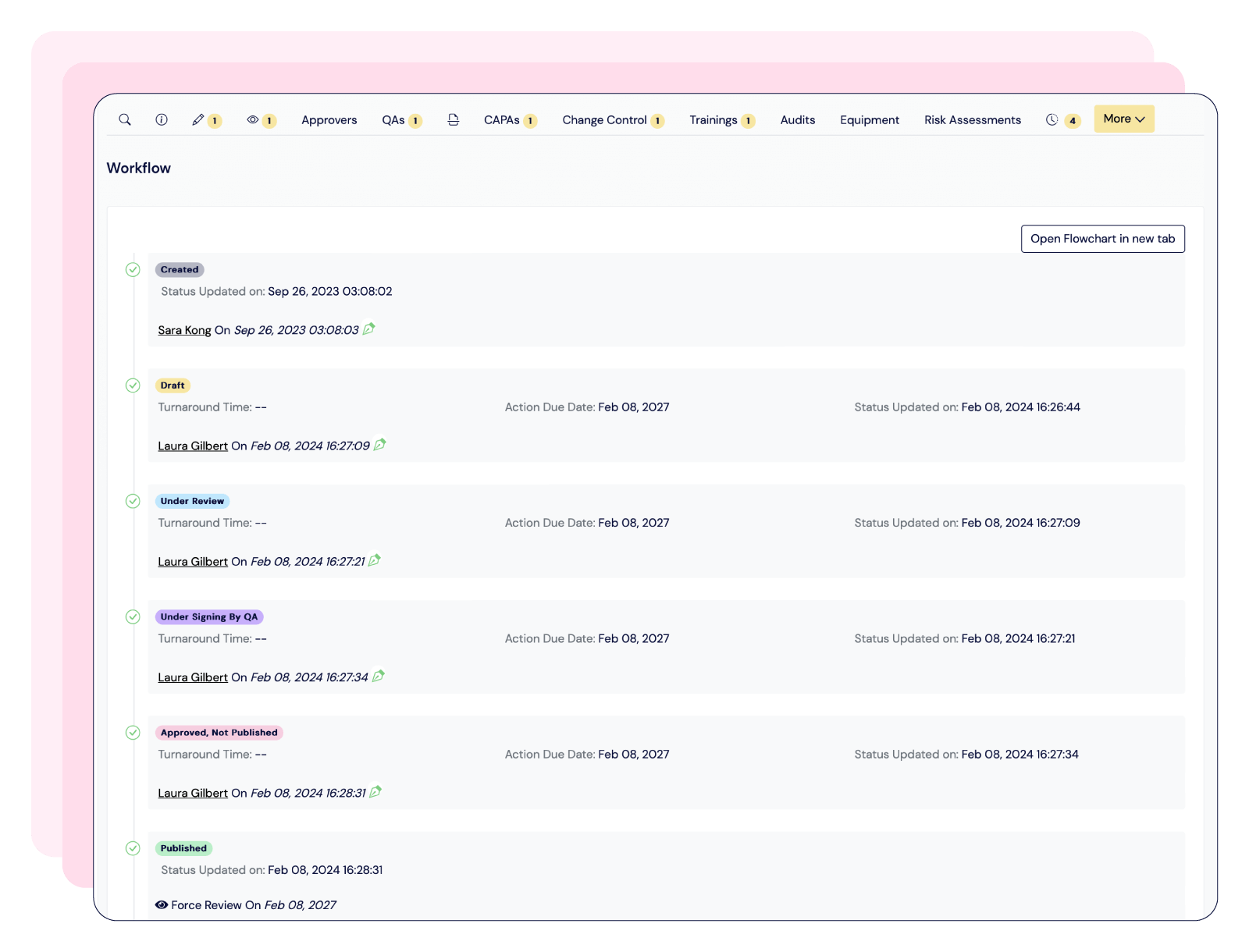
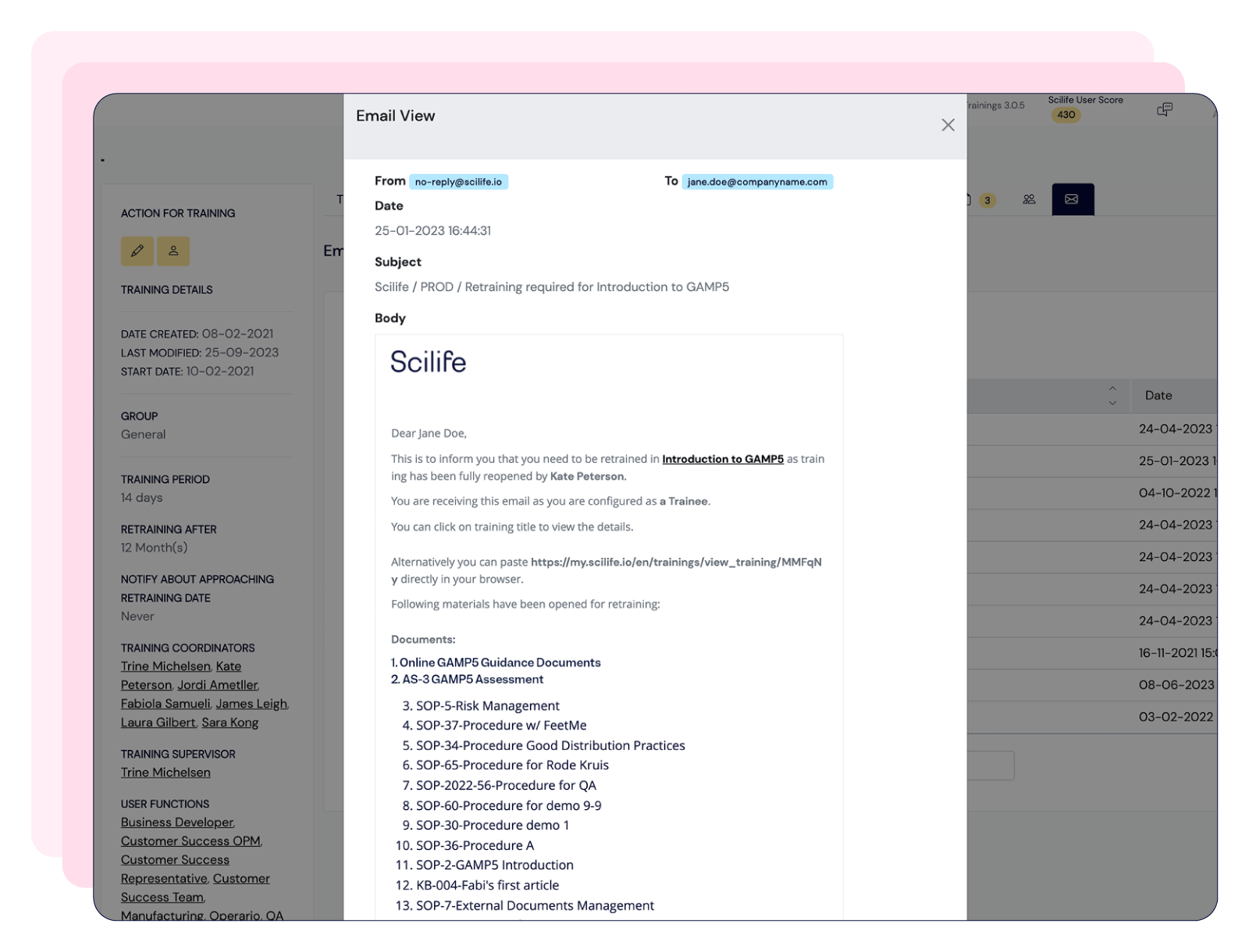
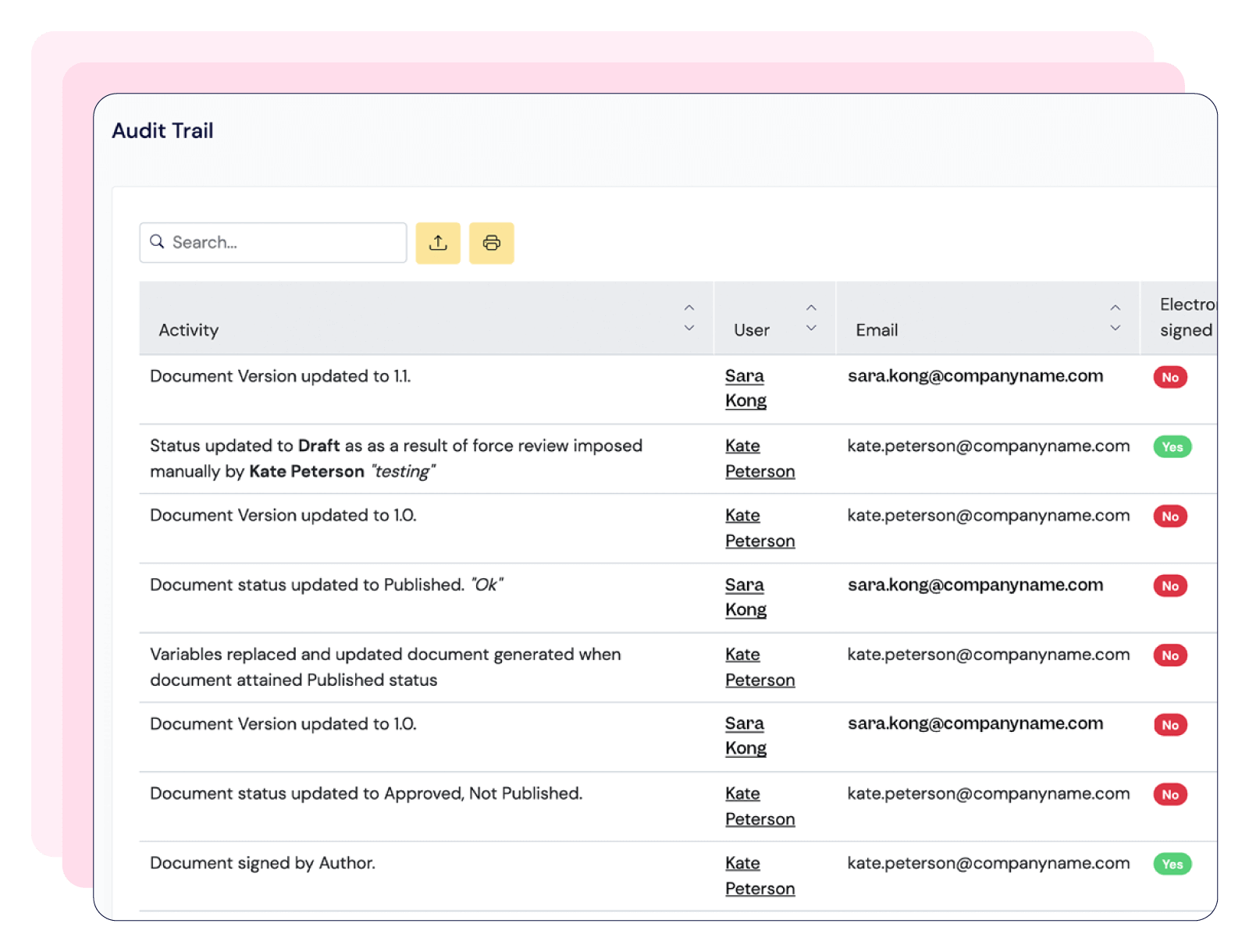
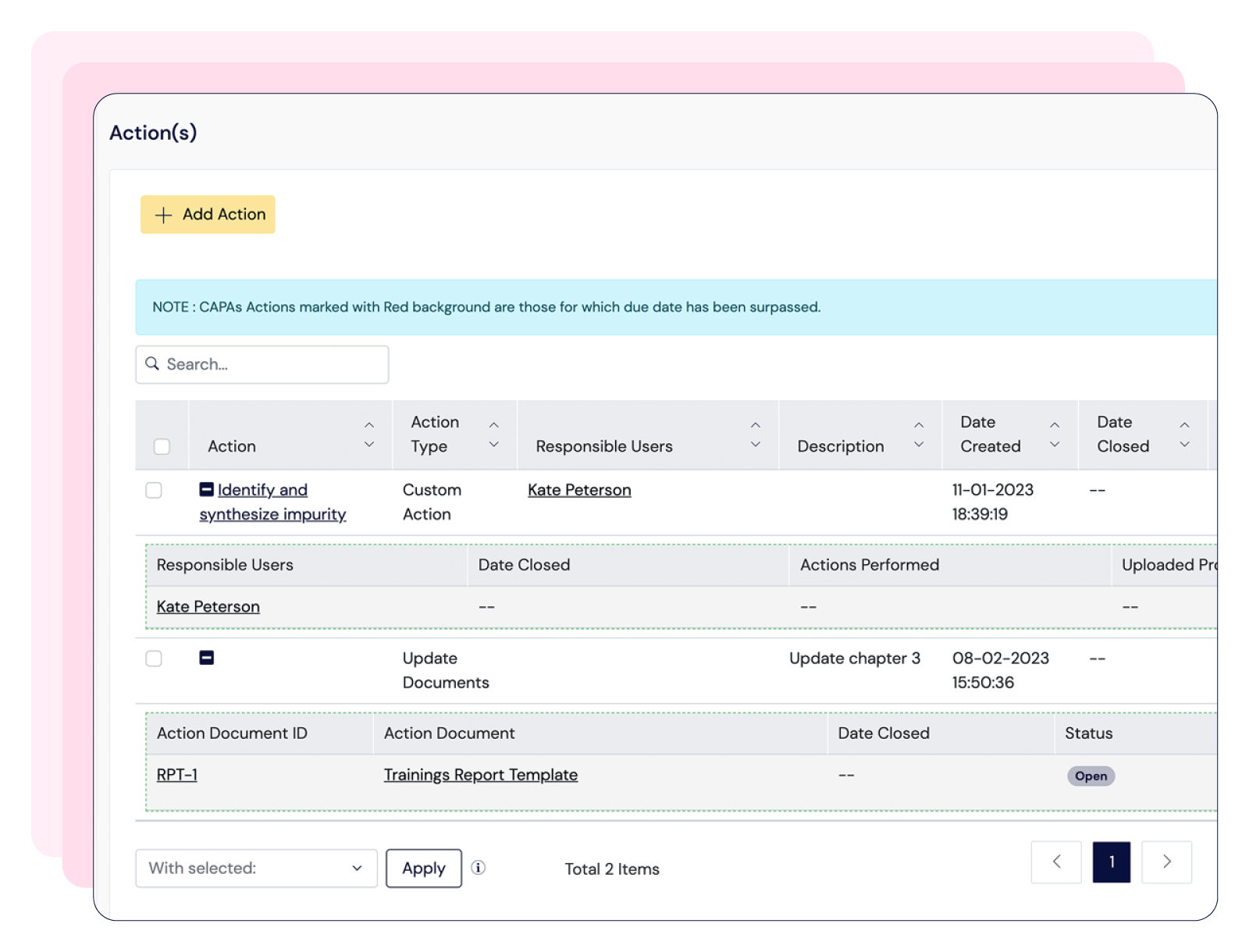
Centralize documentation in compliance with 21 CFR Part 11, streamline training management, and tailor CAPA workflows—keeping your processes audit-ready and fully aligned with GMP standards.
“Scilife has made our life easier in so many ways. One of the things I like the most is how it integrates different processes. It gives a bigger picture of everything and allows us to keep track of deviations and see opportunities for improvement.”
 Daniele Scalco,
Daniele Scalco,
Quality Assurance and Regulatory Affairs Manager at Idevax
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
PRODUCT
INDUSTRIES
RESOURCES
COMPANY
Contact Us
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2025 Scilife N.V. All rights reserved.