Critical Control Point (CCP)
What Is a Critical Control Point?
A CCP is a step in which a control can be applied to slow or stop the growth of microorganisms.
In the Life Sciences space, CCPs are an important part of a system known as Hazard Analysis Critical Control Points (HACCP). HACCP delivers the framework for controlling the pharmaceutical production process, with the goal of lowering the risk of illness and injury in manufacturing.
Every step in the chain that may require pathogen reduction becomes a CCP: pre-processing, storage, shipping, and more. More complex CCPs may include allergen inclusion or even toxic metal detection in pharmaceuticals.
The U.S. Department of Agriculture Food Safety and Inspection Service requires processors to maintain at least one CCP. Generally, this means the CCP will be the point at which the pharmaceutical is most susceptible to microbial growth or other key health and safety issues.
The process is also imperative in regulations such as ISO 22000, which requires manufacturers to meet the minimum human health and safety standards. The benefits of CCP regulations expand throughout the pharmaceutical manufacturing supply chain.
It’s essential to note that CCPs are not a substitute for good hygiene practices. Rather, they should include these practices. In addition, a CCP should consistently focus on control safety over quality issues. Quality controls are crucial as well, but they are a separate entity that must be considered individually.
Key HACCP Terminology
The HACCP process involves a number of concepts in addition to Critical Control Points. These include:
Risk
A risk is the likelihood that conditions will result in a hazard.
Hazard
Hazards involve contamination or microbial growth that could compromise food safety.
Monitoring
Monitoring, in this context, means determining whether the responsible parties have met the CCP criteria.
Severity
Severity assesses how serious the outcomes of a hazard may be.
Critical Limits
Critical limits are the maximum and/or minimum value at which a parameter should be controlled to prevent the food hazard from taking place.
CCPs are based on factors such humidity, acidity, and more. Even sensory information like appearance, smell, and texture play a role.
7 Steps of HACCP
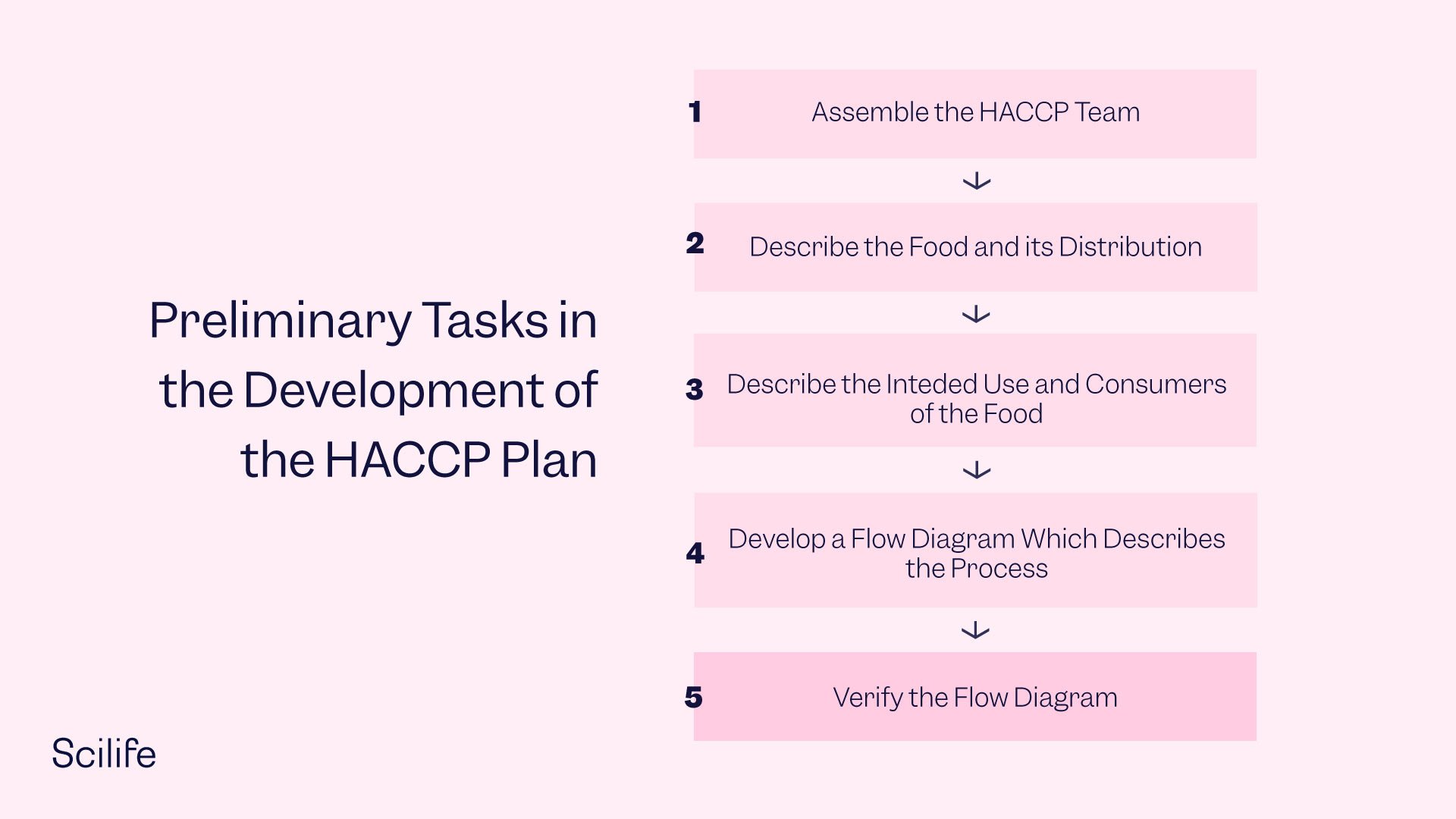
A series of unique steps comprise the HACCP system. Each one helps to address the control of pharmaceutical manufacturing biological, physical, and chemical hazards.
These steps include:
1. Completing a Hazardous Analysis
Here it’s essential to compile a list of hazards that may result in illness or injury without the proper controls. Whether or not the pharmaceutical will be given to vulnerable groups (like the elderly or the immunocompromised), employees’ training and skill levels, the transportation process, level of contact with the pharmaceutical, and storage and transportation are all key considerations.
2. Identifying the CCPs
This is the time to identify and define the Critical Control Points over the course of the supply chain. It’s essential to keep in mind that CCPs—when defined incorrectly or inadequately—may lead to illness or harm. Examples of CCPs include storage, transportation, and distribution. Be sure each CCP outlines the best ways to monitor, measure, and document the pharmaceutical throughout the supply chain.
3. Setting Critical Limits
A critical limit makes sure the hazard—biological, physical, or chemical—is controlled by the CCP. All critical limits should be rooted in science. These limits may come from regulatory guidelines, surveys, or expert findings. Examples of critical limits include time, temperature, chlorination, and other factors. It’s imperative that they can be monitored by observation or measurement (and revisited as needed).
4. Defining Monitoring Procedures
Monitoring procedures feature observations or measurements recorded by a trained professional that determine whether the Critical Control Point is met. They provide written documentation of the flow of pharmaceutical through the facility and across the supply chain. If these procedures reveal the critical limits aren’t met, workers must take measures to comply with the relevant regulatory bodies.
5. Planning Corrective Action
If at any point in the food system professionals struggle to meet the CCP criteria, the responsible party must take corrective action as appropriate. All corrective actions should be based on fact and measurable (as well as documented) so that any gaps or shortcomings can be quickly rectified. An example of a corrective action is to discard the product and store future iterations at a lower temperature.
6. Determining Verification Procedures
Verification procedures go beyond monitoring and assess the validity of the HACCP plan—ensuring the system is functioning so workers can make changes as needed. This is where experts will confirm the plan is science-backed and technically-valid. From there, one can determine all hazards are identified and controlled. Expert advice or even an onsite review of the critical limits may play a role here as well.
7. Establishing Documentation Procedures
All aspects of the HACCP plan should be recorded and documented, with data and visuals included to reflect the relevant standards. Team members should be trained on documentation procedures and understand why recordkeeping is so paramount. Time and temperature logs, flowcharts, staff training records, and standard operating procedures (SOPs) are all documents you’ll want to have on hand.
Conclusion
HACCP is a research-backed tool for mitigating human health hazards in pharmaceutical manufacturing. CCPs are an integral part of this overall strategy. While manufacturers should include at least one Critical Control Point in each of their products, it’s typically most effective to begin with a single product that includes limited hazards. That way Life Sciences organizations can understand clearly what the concept of a CCP entails and elaborate on their strategy moving forward.
Additional resources

How to Implement the Continuous Improvement Cycle | Scilife
Even an organization with stellar leadership and a solid core of employees experiences hiccups from time to time. Despite having assembled all the ...

How to assess and enhance your Quality Management Maturity | Scilife
As the life sciences industry becomes increasingly regulated and competitive, quality management has become more vital than ever. Are you confident ...

Best Quality Management Software (QMS) for Life Sciences | Scilife
The right electronic Quality Management System (eQMS) can help strengthen your compliance processes and build a culture of quality within your ...

How to write a good quality plan for medical devices | Scilife
In life sciences, especially if you’re in the medical device industry it becomes harder to manage projects in accordance with your company’s quality ...
Turn quality into your brightest asset with Scilife
