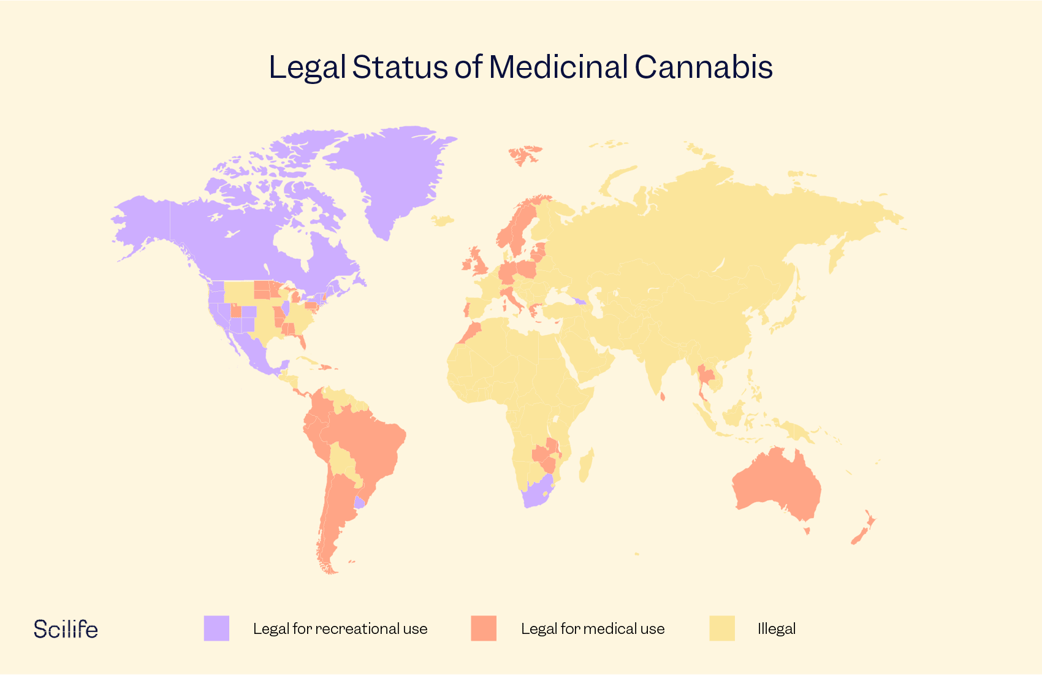
For thousands of years, our ancestors have used the cannabis plant to treat illnesses. The medicinal use of the cannabis flower is made possible thanks to its hundreds of chemical compounds that create pleasant sensations, feelings of relief, and health improvements by interacting with the endocannabinoid system in our bodies. These properties are highly useful for the treatment of patients suffering from a range of illnesses and have recently seen a significant increase in use.
The primary psychoactive component in cannabis is tetrahydrocannabinol (THC), followed by cannabidiol (CBD). While THC and CBD have been the focus of extensive research studies, new properties of other cannabinoids are constantly being discovered. This includes, for example, terpenes and flavonoids, which have also shown therapeutic properties.
Several medicinal uses of cannabis are reported in both traditional or alternative medicine, including Persian Siddha, Ayurvedic, and Unani medicine. Some of the potential modern medicinal uses of cannabis are:
- Chronic pain management
- Alleviating chemotherapy and HIV/AIDS treatment-induced side effects, such as nausea and vomiting
- Epilepsy
- Amyotrophic lateral sclerosis (ALS)
- Huntington’s disease
- Parkinson’s disease
- Dementia
- Traumatic brain injury
- Substitution therapy of opioids, stimulants, and alcohol
- Anxiety
- Post-traumatic stress disorder (PTSD)
- Sleeping disorders
The Events that Stigmatized Medicinal Cannabis
However useful it may be, cannabis can indeed cause drug dependence and is classified as a Schedule I drug by the FDA per the Controlled Drug Substances Act. A long history of events caused this stigmatization of medicinal cannabis:
- The 1961 Single Convention on Narcotic Drugs:
- Cannabis was internationally prohibited and classified in Schedule I of the treaty, which also includes heroin. Schedule I establishes strict controls over substances considered highly addictive and potentially susceptible to drug abuse. It was also added to Schedule IV of the Convention, recognizing the plant’s limited or non-existent therapeutic value.
- The 1961 prohibition faced opposition from countries like India, Mexico, Myanmar, and Pakistan, where traditional use of Cannabis is quite common. India played an important role in keeping references to leaves and seeds of cannabis out of the 1961 convention, which is why India maintains the traditional and religious use of Bhang, an edible preparation made from the leaves of the cannabis plant.
- The 1971 Convention on Psychotropic Substances:
- THC was listed in Schedule I as a substance for which control is recommended because:
- It may be abused, posing a severe risk to public health; and
- Its therapeutic value is very limited or inexistent.
- The 1988 United Nations Convention against the Illicit Traffic of Narcotic Drugs and Psychotropic Substances classified the following activities as criminal offenses:
- The production, manufacture, supply, distribution, sale, transportation, import, or export of any narcotic drug or psychotropic substance and the cultivation of the cannabis plant to produce drugs; and
- Possession or acquisition of any narcotic drug or psychotropic substances for any of the purposes mentioned above.
However, it is worth noting that cannabis consumption was excluded from the list of criminal behaviors at that time. Nevertheless, any use of cannabis was naturally stigmatized because of this series of events. Soon, medical and scientific research started lagging behind due to dramatically increased criminal justice interventions.
Breaking the Stigma
Due to the stigmatization, all cannabis research was kept under wraps. However, gradually awareness of the medicinal use of cannabis grew, and voices in favor of legalizing medicinal cannabis became louder. Today, many countries around the globe are starting to legalize medicinal cannabis and research related to it. Let’s look at how these steps are being implemented across the world:
Medicinal Cannabis in North America
The medicinal cannabis industry in the United States and Canada is already relatively advanced:
Medicinal Cannabis in the United States
In the US, different legislative frameworks exist in different states. In 2021, the National Organization for the Reform of Marijuana Laws (NORML) successfully lobbied for many cannabis-related reforms across different states. Some of these latest legislative updates are described below:
Medicinal Cannabis in Alabama
Senate Bill 46 legalizes registered patients to possess up to 70 medical marijuana daily dosages of a maximum of 50 milligrams at a time, effective since May 17, 2021. According to this bill, patients must obtain cannabis from a licensed dispensary in the form of:
-
-
- Tablets, capsules, tinctures, gel cubes for oral use;
- Gels, oils or creams, transdermal patches for topical use;
- Suppositories for parenteral use; or
- Nebulizers, liquids, oils for use in an inhaler.
Medicinal Cannabis in Arkansas
- Senate Bill 654 bill extends the period for which a patient visiting from another state can legally access cannabis medicines in Arkansas to 90 days.
Medicinal Cannabis in California
- Senate Bill 311 allows terminally ill patients to use medicinal cannabis within a healthcare facility. It prohibits patients from inhaling or vaping herbal cannabis products and restricts the use of any forms of cannabis in emergency rooms effective since January 1, 2022.
Medicinal Cannabis in Colorado
- Senate Bill 56, effective since May 6, 2021, requires school boards to implement policies to allow the storage, possession, and administration of cannabis-based medicines by school staff. It also allows school staff to volunteer to possess, administer, or assist in administering cannabis-based medications. It additionally protects those subjects from retaliation.
Medicinal Cannabis in Delaware
- Senate Bill 60 enables physicians, assistants, and nurse practitioners to issue medical cannabis authorizations effective since June 15, 2021.
Medicinal Cannabis in the District of Columbia
- PR24-0456 provides a grace period for registered patients to continue to access medical cannabis without renewing their authorization.
Medicinal Cannabis in Georgia
- Senate Bill 195 permits the establishment of up to 30 state-licensed retailers of high-CBD/low-THC oil products effective since July 1, 2021.
Medicinal Cannabis in Louisiana
- House Bill 391 includes raw or crude cannabis for inhalation in licensed cannabis medicinal products. Effective since January 1, 2022, it also allows registered patients to purchase up to two and a half ounces of medical cannabis flower for a 14-day period from licensed providers.
Medicinal Cannabis in Minnesota
- House File 2128 includes cannabis flowers in licensed medicinal products. The law was signed on May 25, 2021.
Medicinal Cannabis in New Hampshire
- House Bill 89 expanded the pool of qualifying conditions for medical cannabis to include moderate to severe insomnia and autism spectrum disorders. It took effect on June 24, 2021.
- House Bill 605 includes opioid use disorder in the list of qualifying conditions for the prescription of medicinal cannabis. Effective since October 9, 2021, it also permits on-prescription out-of-state residents to purchase cannabis in the state.
Medicinal Cannabis in New Jersey
- House Bill 89 expanded the pool of qualifying conditions for medical cannabis to include moderate to severe insomnia and autism spectrum disorders. It took effect on June 24, 2021.
- Senate Bill 619 permits healthcare practitioners to authorize any qualifying patient for the medical use of cannabis via a telehealth visit starting June 24, 2021.
Medicinal Cannabis in North Dakota
- House Bill 1359 increases the number of caregivers for an authorized medical cannabis patient and eliminates the accompanying $50 application fee effective since August 1, 2021.
Medicinal Cannabis in Oregon
- Senate Bill 307 waives application fees for certain veterans seeking authorization in the state’s medical cannabis access program, effective since January 1, 2022.
Medicinal Cannabis in Pennsylvania
- House Bill 1024, effective since June 30, 2021, expanded the quantities of cannabis that authorized patients may possess beyond 90 days. The law includes cancer remission therapy and CNS-related neuropathy as eligible conditions to prescribe medicinal cannabis while eliminating provisions that previously required chronic pain patients to try conventional prescription pain medications before using cannabis.
Medicinal Cannabis in Texas
- House Bill 1535 expanded the Texas Compassionate Use Program (TCUP) by allowing patients with PTSD and all cancer types to access low-THC cannabis products. It also raises the cap on legally regulated THC products from 0.5% to 1%, effective since September 1, 2021.
Medicinal Cannabis in Utah
- Senate Bill 170 expanded the pool of medical professionals who can recommend medical cannabis to qualified patients. It also allows patients to access their medicine while their application is still under review, effective since March 17, 2021.
Medicinal Cannabis in Virginia
- House Bill 2218/Senate Bill 1333 amended the state’s medical cannabis access law to allow botanical or whole-plant medical cannabis products to be produced and dispensed from July 1, 2021, forward.
- House Bill 1988 permits patients in hospice and other residential facilities to access medicinal cannabis products. It also enables patients to obtain medical cannabis authorizations via telehealth appointments effective July 1, 2021.
- House Bill 1862, effective on July 1, 2021, prohibits employers to discharge, discipline, or otherwise discriminating against employees for the lawful use of medical cannabis while away from the job.
- House Bill 2218/Senate Bill 1333 amended the state’s medical cannabis access law to allow botanical or whole-plant medical cannabis products to be produced and dispensed from July 1, 2021, forward.
In Canada, 44 licensed producers are authorized by the Ministry of Health, and thousands of Canadians are licensed to possess and consume medicinal cannabis. In both cases, self-cultivation is allowed if it does not exceed six plants and its use can be justified.
Medicinal Cannabis in Canada -
Medicinal Cannabis in Europe
The last few years have led to positive—but cautiously limited—steps across Europe:
Medicinal Cannabis in The Netherlands
-
- The use of medicinal cannabis has been permitted since 2003. The Dutch framework is an example of a long-established system that provides access to medicinal cannabis.
-
- All activities related to medicinal cannabis are strictly regulated, and the Dutch Office of Medicinal Cannabis (BMC) has complete control.
- The regulatory framework was led by the Dutch company Bedrocan Medical Cannabis, which holds the monopoly on all medicinal cannabis production and distribution in the Netherlands.
- All cannabis passing through the BMC is produced by Bedrocan, which developed and standardized domestic demand and exported some of the five types of pharmaceutical cannabis flower medications prepared with different percentages of THC and CBD.
- Medicinal cannabis is produced nationwide and controlled by the Dutch Medicinal Cannabis Agency.
- Products can be purchased in pharmacies for several pathologies only when the patient has a medical prescription.
- Medicinal use has increased dramatically over the past decade, with over 50,000 patients now being prescribed cannabis in the Netherlands.
Medicinal Cannabis in Greece
- Greece legalized medical cannabis in 2017 and revoked a ban on cultivation and production in March 2018.
- On March 1, 2018, Greece adopted the Provisions for the Production of End Products of Medicinal Cannabis Bill that proposed to allow:
- Patients to access medicinal cannabis products for specific illnesses; and
- Individuals to cultivate cannabis to produce medicinal products in the country.
- Patients to access medicinal cannabis products for specific illnesses; and
Medicinal Cannabis in Poland
- Medicinal cannabis was legalized in July 2018 by the Piotr Liroy-Marzec Bill, effective since November 2018.
- Doctors can prescribe medicinal cannabis for any disease, provided this is backed by sufficient research.
- The law only allows cannabis import, which takes place mainly from the Netherlands rather than domestic production or self-cultivation.
Medicinal Cannabis in Slovenia
- Cannabis is transferred from Group I to Group II on the National List of Illicit Substances.
- As of February 2018, the Decree on the Classification of Illicit Drugs (Official Gazette of the Republic of Slovenia, #45/14, #22/16, and #14/17) allows medical practitioners to prescribe cannabinoid-based medications (categorized as synthetic, natural, and medicinal cannabis), as well as standardized buds and flowering tops of cannabis (although the latter remains to be fully implemented in practice).
- All activities related to medicinal cannabis are strictly regulated, and the Dutch Office of Medicinal Cannabis (BMC) has complete control.
Medicinal Cannabis in Germany
-
- Germany completed the legislative reforms necessary to expand the medical use of cannabis in January 2017.
- Before 2017, patients could only gain access to medical Cannabis through a special individual authorization.
- It is now one of the first countries in the world to include medical cannabis in its basic list of medications covered by both private insurers and public health services.
- The German Cannabis Agency was established under the Federal Institute for Drugs and Medical Devices to oversee the new process, as determined by international drug treaties.
- The 2017 law also allows for the domestic production of Cannabis; although, for now, all cannabis products continue to be imported, mainly from the Netherlands.
- Germany completed the legislative reforms necessary to expand the medical use of cannabis in January 2017.
-
Medicinal Cannabis in the United Kingdom
- The government only permits the use of Sativex for patients with multiple sclerosis under medical prescription.
- The National Health Service (NHS) has established that every patient must pay for the medication about 500 EUR (428 GBP) a month.
- The steep price is a challenge for many patients.
- The government only permits the use of Sativex for patients with multiple sclerosis under medical prescription.
Medicinal Cannabis in the Czech Republic
-
- Medical cannabis was legalized in 2013.
- There is no straightforward process for acquiring licenses to produce, sell, or purchase products derived from cannabis. Hence, there continues to be uncertainty about the scope and potential of this reform.
Medicinal Cannabis in Denmark
- Cannabis for therapeutic purposes is still illegal, but a pilot program began on January 1, 2018, allowing doctors to prescribe new cannabis products that were not legal in Denmark before.
- The pilot program will run until December 31, 2025.
- Currently, available cannabis products can be seen in medicine prices.
- Medical cannabis was legalized in 2013.
Medicinal Cannabis in Ireland
-
- Patients can acquire medical cannabis via prescription under the official Medicinal Cannabis Access Program.
- Prescriptions are reserved for cases where standard treatment has previously failed, including specific conditions such as multiple sclerosis, intractable nausea, vomiting associated with chemotherapy, and severe refractory (treatment-resistant) epilepsy.
- Patients can acquire medical cannabis via prescription under the official Medicinal Cannabis Access Program.
Medicinal Cannabis in Israel
Israel is an exception in the Middle East, as it approved the medicinal use of cannabis in 1992 and soon became a center for scientific research and development of cannabis varieties and industrial products. Legislation is implemented by a specially established unit in the Ministry of Health, the Israeli Agency on Medical Cannabis (IMCA), which established a steering committee in collaboration with the Israeli police, the Ministry of Agriculture, and the Ministry of Economy. The IMCA issues different license types for the cultivation, extraction, packaging, and distribution of cannabis. It is also responsible for authorizing clinicians to prescribe cannabis to patients suffering from severe pain and other symptoms. In addition, other illnesses can be treated in hospitals as part of clinical trials. By 2017, some 40,000 patients were receiving medicinal cannabis in Israel.
Medicinal Cannabis in Asia
In Asia, medicinal cannabis is strictly prohibited in Japan, Vietnam, Pakistan, Cambodia, and Nepal, while there is some progress in India, the Philippines, and Thailand:
Medical Cannabis in India
-
- The law defines two types of cannabis products:
- Ganja (the flowering or fruiting tops of the cannabis plant), which has slightly relaxed regulations; and
- Charas or hashish (cannabis resin).
- Ganja (the flowering or fruiting tops of the cannabis plant), which has slightly relaxed regulations; and
- India already has some legal provisions for the medicinal and scientific usage of the plant, but these provisions have yet to be implemented.
- Since 2017, various political figures, including Maneka Gandhi and MP Dr. Dharamvir Ghandi, have shown their support for cannabis policy reform.
- The 2015 Phytopharmaceutical Act was passed to accelerate investigations on plant-based medicines – a move that is expected to attract cannabis research investments from multinational corporations.
Medicinal Cannabis in the Philippines
- The House Committee on Health approved the Medical Compassionate Medical Cannabis Act in September 2016, which prohibits the use of raw cannabis.
- Patients need prior authorization from a doctor and treatment needs to be delivered in dedicated hospitals with a special license from the Department of Health.
- The Philippine Drug Enforcement Agency is responsible for regulating and dispensing medicinal cannabis, which can be used to treat various ailments, including arthritis, epilepsy, and multiple sclerosis.
- The bill also plans to create a research facility on medicinal cannabis.
- The law defines two types of cannabis products:
-
Medicinal Cannabis in Thailand
- A public forum was held in August 2016 to remove cannabis from Category 5 of the country’s drug list.
- The Agricultural Council was tasked to develop a proposal for the decriminalization of the substance for consideration by the government.
- Starting January 1, 2017, hemp was decriminalized in 15 districts and six provinces of the northern region.
- A public forum was held in August 2016 to remove cannabis from Category 5 of the country’s drug list.
Medicinal Cannabis in Australia and New Zealand
Recent years have seen significant developments in Australia and New Zealand:
Medicinal Cannabis in Australia
-
- A national body can issue licenses to growers and regulate medicinal cannabis crops for the cultivation of medicinal marijuana.
- Medical practitioners can supply medicinal cannabis to a patient after notifying the regulatory authority and after receiving permission from the state or territory government.
- Medicinal cannabis can also be used for clinical trials.
- The state of New South Wales first allowed wide-ranging medicinal cannabis trials and provided police with the power to not prosecute terminally-ill patients using cannabis. A small number of children with the worst case of drug-resident epilepsy can also be prescribed medicinal marijuana under a compassionate access scheme.
- Victoria was the first state to establish a state-based medicinal cannabis scheme, allowing children with severe epilepsy to be provided with the drug. All other states and territories have schemes to enable access to medicinal cannabis via prescription for various conditions.
- Queensland published the first guidance documents for health practitioners in March 2017. In December 2017, Australia produced national guidelines for five conditions and published these on the nation’s Therapeutic Goods Administration website.
- A national body can issue licenses to growers and regulate medicinal cannabis crops for the cultivation of medicinal marijuana.
-
Medicinal Cannabis in New Zealand
- The Misuse of Drugs Amendment Bill in December 2017 made medicinal Cannabis available without criminal liability.
Medicinal Cannabis Reforms in Latin America and the Caribbean
Latin America is currently leading the way for the adoption of policies and easy access to cannabis for therapeutic uses.
Medicinal Cannabis in Uruguay
-
- This is the first country to completely legalize the cannabis market for scientific, industrial, and recreational purposes.
- The Institute for Regulation and Control of Cannabis determines who can produce and consume cannabis as well as how much and under which conditions.
- The regulatory framework for recreational use is based on issuing licenses to individuals interested in planting, growing, harvesting, producing, and commercializing cannabis.
- There are three forms of access: self-cultivation for personal use, cannabis clubs, and purchases made in pharmacies. These are mutually exclusive, and the amount of cannabis that can be acquired is limited to 40 grams (1.4 ounces) a month per person.
- A patient who needs to purchase medicinal cannabis needs to request an Orange Prescription Form, which is the most restricted prescription type. If the application is accepted, the cost of medicinal cannabis remains exceptionally high.
- This is the first country to completely legalize the cannabis market for scientific, industrial, and recreational purposes.
-
Medicinal Cannabis in Chile
- Patients in need of medical cannabis can access it with the help of a medicinal prescription (Decree 84 of the Institute for Public Health).
- The authorized import of cannabis-based medications is possible under exceptional circumstances with the approval of the National Medical Regulatory Agency.
- Patients in need of medical cannabis can access it with the help of a medicinal prescription (Decree 84 of the Institute for Public Health).
-
Medicinal Cannabis in Colombia
- In Colombia, the state controls the market and grants licenses to private entities for the production, manufacture, export, and research of cannabis for scientific and medicinal uses under Law 1787, approved in 2015.
-
Medicinal Cannabis in Jamaica
- In Jamaica, medicinal cannabis must be recommended or prescribed by a registered physician or a health professional certified by the Ministry of Health.
- Tourists and non-residents in Jamaica can apply for a permit to purchase and possess up to two ounces of cannabis. They must present a doctor’s prescription or simply sign a declaration stating their medical condition for the application.
- In Jamaica, medicinal cannabis must be recommended or prescribed by a registered physician or a health professional certified by the Ministry of Health.
-
Medicinal Cannabis in Brazil
- The import of medications based on CBD oil, THC, and marijuana flowers has been legal since 2016.
- However, the Brazilian Federal Medical Board prohibits marijuana prescriptions in its natural form unless there are exceptional circumstances.
- Import requires compliance with a series of requirements established by the National Health Surveillance Agency. These include patient registration, handling administrative procedures for import in-person, and applying for a permit from the agency.
- The import of medications based on CBD oil, THC, and marijuana flowers has been legal since 2016.
-
Medicinal Cannabis in Argentina, Peru, and Mexico
- Argentina, Peru, and Mexico have adopted other, less ambitious, regulatory processes. In these three countries, reforms resulted from active societal pressure and patient groups, leading to policies allowing for the sale and use of medicinal cannabis in October 2017.
-
Medicinal Cannabis in Bolivia
- Bolivia amended its drug legislation on March 16, 2017, to allow medicinal cannabis. Placing it within the framework of broader drug legislation, individuals and companies must register and request prior authorization from the Ministry of Health to import, export, trade, or produce medicinal cannabis. The ministry may also grant exceptional and limited authorizations for research on medicinal cannabis.
Conclusion
Globally, the legislative framework for the justified medicinal use of cannabis is still evolving as the world is gradually developing a more mature and scientific outlook on medicinal cannabis. Nevertheless, we hope that we were able to give you a high-level overview of the rules, regulations, and complications of the medical cannabis industry.
Do you want to know how Scilife can help you become totally compliant? Discover Scilife Smart Quality Platform for Medicinal Cannabis!
Curious about how medical cannabis companies are dealing with regulations? Access the Scilife case study on Helius Therapeutics here.


