Staying ahead of industry shifts has never been more critical for anyone working in biotech, pharma, or MedTech. The pace of innovation is accelerating, regulatory expectations are tightening, and AI is reshaping everything from discovery to commercialization.
As we step into 2026, we’re seeing a new wave of emerging trends in life sciences; real forces already influencing strategy, investment, and day-to-day operations across the whole ecosystem. These life sciences industry trends cut across the value chain and are shaping how organizations plan, build, and compete.
In this article, I’ll walk you through the most important developments to watch in 2026, drawing from what we’ve been hearing in the field, what analysts are predicting, and where companies we know are already placing their bets.
I’ll also give you actionable steps on how you can position your organization to move with the momentum of 2026.
Key takeaways
Below are the most important life sciences industry trends shaping 2026, along with practical actions you can take now to stay ahead.
Emerging life sciences industry trends in biotech and pharma
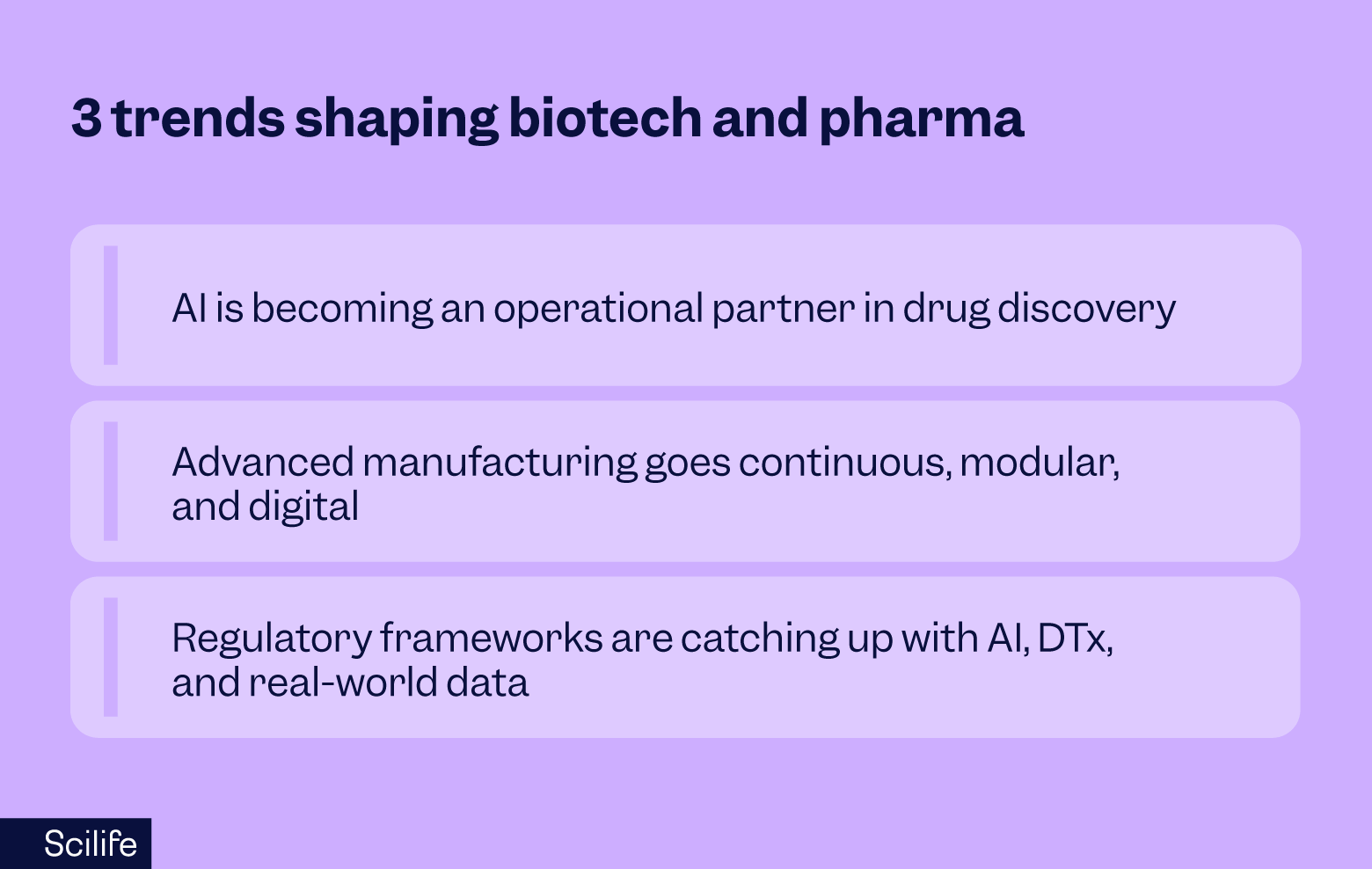
Within the broader set of life sciences industry trends, I keep noticing the same handful of forces converging in biotech and pharma – AI advancing as an operational partner, advanced manufacturing, and the concurring evolution of the regulatory framework.
Get ready for reading about some of the most defining pharma industry trends and biotech industry trends shaping 2026.
Here’s what I’d be watching closely if I were in the field today.
AI becomes an operational partner in drug discovery (not just a buzzword)
No surprise to see AI among the biggest life sciences industry trends.
2026 is shaping up to be the year AI finally shifts from “experimental pilot” to a real, day-to-day partner for discovery teams. I’m seeing pharma organizations build their own high-performance computing environments, expand long-term collaborations with AI-native biotech, and quietly celebrate noticeably shorter design-to-lab cycles, such as Nabla Bio and Takeda’s partnership that can reportedly go from design to lab testing in 3-4 weeks.
It’s no longer about proving AI works; it’s about figuring out how to scale it. This shift is one of the most impactful pharma industry trends changing how teams work.
Quick action:
Choose one discovery function (target prediction, molecule design, or translational modeling) and launch a small, clearly measurable AI pilot to build internal confidence and capability.
Recommended learning:
More on AI in drug development – read use cases and latest trends.
Automated compliance workflows as a trend in operational excellence
As life sciences organizations adopt AI and advanced digital tools, compliance processes must evolve at the same pace. Automated compliance workflows are emerging as a key driver of operational excellence, enabling teams to embed regulatory requirements directly into everyday processes. This shift allows companies to move faster without increasing risk, ensuring compliance is maintained by design rather than enforced retroactively.
Advanced manufacturing - continuous, modular, and digital
Pharma manufacturing is stepping into a new era. Continuous processing, modular setups, and smart PAT (process analytical technology) systems are moving quickly from innovation labs into real-world production. These approaches don’t just make plants more efficient - they shrink footprints, increase flexibility, and help teams scale both small molecules and biologics faster than ever before.
From my conversations with manufacturing teams, the excitement isn’t just about speed; it’s about having the ability to pivot quickly when demand or product needs change.
Real-world example: According to the results of CRB’s Horizons: Life Sciences survey released in October 2024, 75% of respondents are using or plan to use continuous technologies almost exclusively within the next five years, based on a survey of 500 industry leaders, including therapeutic developers and CDMO executives.
Quick action:
Look at your highest-value products and ask where continuous processing or digital twin simulations could save time, reduce risk, or improve scale-up.
Regulatory frameworks evolve around AI, digital therapeutics, and real-world data
2026 will see regulators providing more guidance and running pilot programs for AI/ML tools, algorithm change management, and novel therapeutic modalities. Regulators are also getting much clearer on how real-world data can be used and their expectations for monitoring model drift, validating updates, and demonstrating ongoing safety and efficacy.
Companies that embed regulatory thinking and real-world data into product development early are far more prepared when it comes time for submissions.
Quick action:
Integrate regulatory requirements into product roadmaps from day one. Build workflows that prioritize data provenance and carefully document every step in your real-world evidence analyses to make the data regulatory-ready.
Emerging life sciences industry trends in medical devices
For MedTech in 2026, and within the broader set of emerging trends in life sciences, I believe we’re entering a phase where smart design, digital health, and resiliency are not just “nice-to-have” innovations, but fundamental to staying competitive.
These shifts represent some of the most important medical device industry trends shaping the next few years.
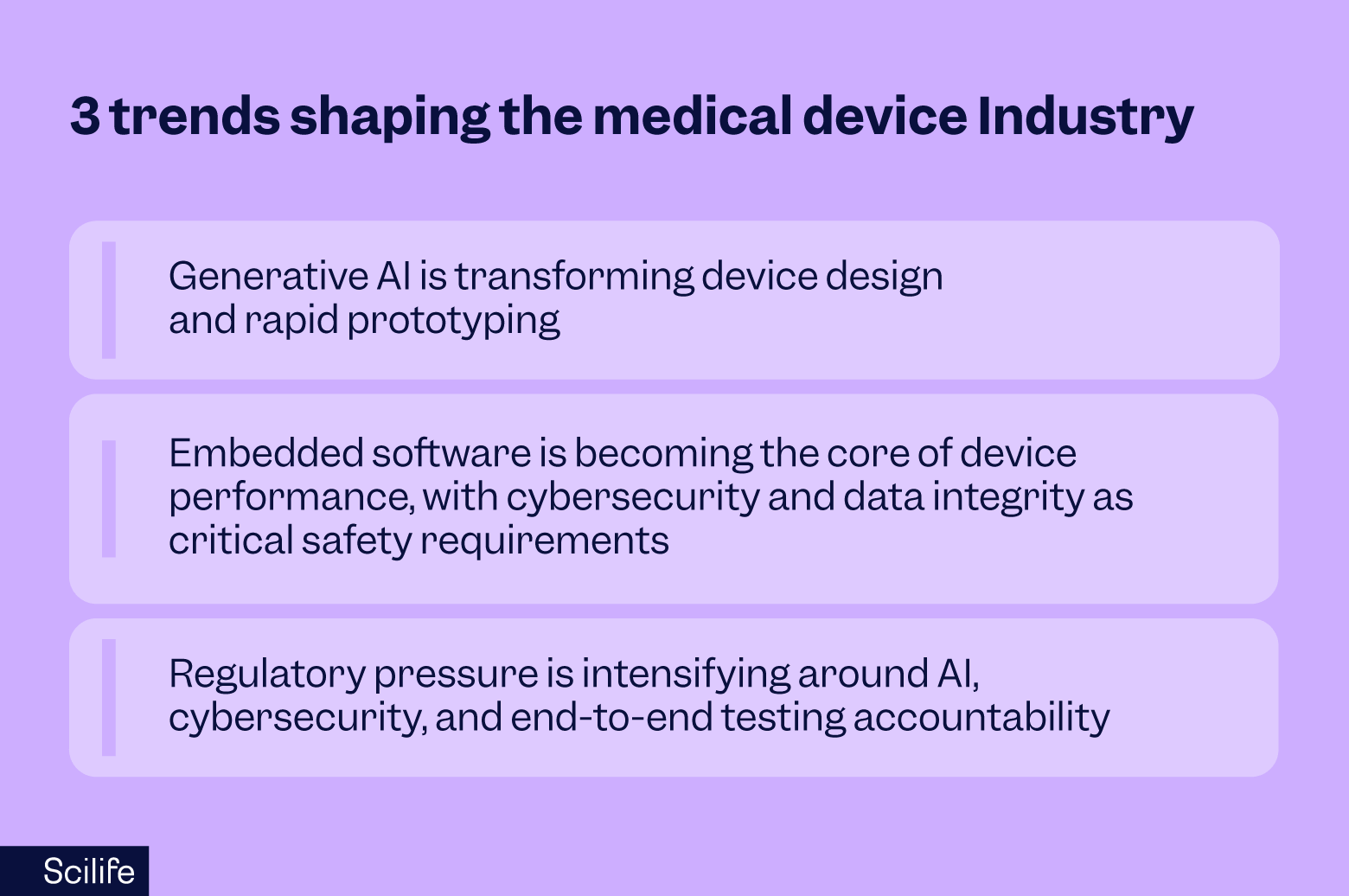
Generative AI supercharges device design and prototyping
One of the most exciting medical device industry trends is how generative AI is transforming device design. Instead of spending weeks manually iterating in CAD, engineers can now generate hundreds of optimized design variations in a single session. These designs account for performance, structural integrity, weight, and manufacturability.
This doesn’t replace engineering expertise; it amplifies it. The real challenge now is keeping validation in step with design: simulation-driven testing, automated regression checks, and high-volume verification processes are becoming essential.
Quick action:
Start building test frameworks that can keep pace with rapid design iteration to make sure generative AI truly accelerates your workflow.
Recommended learning:
Embedded software is now the heart of device functionality – and thorough cybersecurity programs and data integrity become non-negotiable
Even devices traditionally thought of as hardware-first now rely heavily on embedded software for connectivity, analytics, and real-time decision support. Firmware updates and continuous improvement are expected as part of the product lifecycle. As devices become more connected, their attack surface grows. Cybersecurity failures are no longer just IT concerns - they can impact patient safety.
This shift demands mature software development pipelines, including versioning, traceability, automated regression testing, and detailed documentation. Security testing is expanding to include penetration testing, adversarial simulations, and even digital twin-based threat modeling.
Failures in embedded software and/or cybersecurity measures are a growing cause of recalls, and regulators are paying close attention. Dual compliance with both the EU MDR and the EU AI Act makes good documentation practices and traceability more important than ever.
Quick action:
Invest in strong software quality processes to reduce risk and ensure compliance with regulatory expectations. Treat cybersecurity like a core safety requirement. Design it into devices from the start and validate continuously, not just during annual reviews.
Regulation intensifies - especially around AI, cybersecurity, and testing accountability
2026 is shaping up to be a year of heightened regulatory scrutiny. High-risk AI systems require formal conformity assessments, cybersecurity expectations are rising across connected devices, and testing and validation pipelines must be transparent and traceable.
From conversations with regulatory experts, one thing is clear: compliance is no longer a final step. It’s a continuous part of the engineering process and will be checked at every step of a device’s lifecycle.
Regulatory bodies explicitly expect AI-driven MedTech to embed robust quality, safety, and transparency controls into their devices, as became evident when the Medical Device Coordination Group (MDCG) published the guidance document MDCG 2025-6: FAQ on Interplay between the Medical Devices Regulation & In vitro Diagnostic Medical Devices Regulation and the Artificial Intelligence Act in June 2025.
Quick action:
Embed regulatory checkpoints at each stage of design and development to make compliance an integral part of your workflow.
Emerging trends across life sciences industries
Some of the most impactful life sciences industry trends aren’t confined to biotech, pharma, or MedTech – they span the entire ecosystem and reshape how the industry operates as a whole.
Of all the shifts happening in 2026, this is the one I’m watching most closely.
Sustainability becomes a strategic pillar, not a side project
Environmental impact is no longer optional. Manufacturers are expected to minimize waste, use renewable or recyclable materials, and design products that last or can be refurbished. Circular design principles, like modular parts, easy disassembly, sustainable packaging, and replaceable components, are gaining traction.
Manufacturing partners are being evaluated based on sustainability, not just cost. Leading teams are treating sustainability as a core design and operational metric.
Quick action:
Start incorporating lifecycle analysis into product design and embed sustainability metrics into procurement and supplier selection.
Recommended learning:
How your QMS can contribute to environmental sustainability.
Final thoughts on emerging life sciences industry trends
From where I stand, 2026 feels like a turning point for the entire life sciences industry. We’re not just improving drugs or devices - we’re reimagining how they’re discovered, designed, manufactured, validated, and delivered to patients. The convergence of biotech industry trends, pharma industry trends, and medical device industry trends is setting a new standard for innovation.
As the landscape evolves, having a clear path forward matters. Taken together, these life sciences industry trends point to a fundamental shift in how innovation, quality, and compliance intersect across the sector.
Partnering with Scilife can help teams and organizations translate these emerging life sciences industry trends into practical strategies, navigate regulatory complexity, and turn the challenges of 2026 into opportunities for growth.