EMEA Office
Louizalaan 489
1050 Brussels
Belgium
Transform the way you manage complaints:
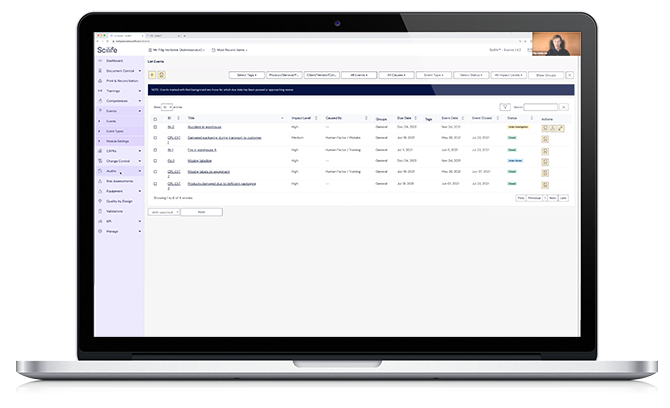
In this free session, Angel Buendía (Knowledge Manager) and Jordi Ametller (Product Specialist Lead) will show you how Scilife’s Complaint Management Solution can help you improve the way you handle complaints within your organization.
In this session, we’ll see a practical demonstration of how to:

Knowledge Manager
at Scilife

Product Specialist Lead
at Scilife
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
PRODUCT
INDUSTRIES
RESOURCES
COMPANY
Contact Us
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2026 Scilife N.V. All rights reserved.