EMEA Office
Louizalaan 489
1050 Brussels
Belgium
00 : 03 : 15
From an academic idea to launching an industrial product under CE
00 : 14 : 00
Challenges in Sales
00 : 21 : 50
Getting ready for ISO 13485
00 : 28 : 20
First large contracts
00 : 44 : 30
From an R&D company to a Sales Company and OraSure subsidiary
Watch this exciting journey from academic research to acquisition and beyond with Vanessa Vankerckhoven, CEO and co-founder of Novosanis. Novosanis is a medical device company and a subsidiary of OraSure Technologies Inc, a leader in point of care diagnostics and molecular collection devices.
In this free session, you will learn about:
Q: Do you have consultants that can help others sponsor in regulatory, ISO certifications, etcetera?
A: Yes, we still use consultants if that's the question. We still heavily rely on external resources, and they help, even though we have grown the team. And of course, under the wings of OraSure, we now have a fully-fledged regulatory quality team. But yet, I think the external consultants still offer additional and added value in the process.
Q: Is Scilife also part of OraSure?
A: No, is not. Novosanis became Scilife’s customer and then Novosanis was acquired by OraSure, and instead of them dictating which system should Novosanis use, they were opened to see what is working best for their quality system. They ended up implementing Scilife in all the families of companies within the group.
Q: Can you share a plan and strategy to increase Novosanis market share in the Asian-African market?
A: Yeah, sure. I think what we had been doing initially, especially being a smaller company was to really build on in-house sales, direct sales models. And what we're doing right now is to build more of a distribution network.
And I think the good thing about being part of the OraSure family of companies now is indeed, that regulatory support we're getting because if you want to move into other markets, that's a big challenge in the medical device industry as well.
You need to get your product registered in all those different countries to CE marking as such is not sufficient, you need all of those local registrations, for instance, for Indonesia, Vietnam, Thailand, and that's of course, where regulatory affairs come into our interplay because they really support those product registrations. And that's the only way I think, to really move in and establish market share into those territories, and we're actively executing on that for sure.
Q: What are your views on the new Flanders health tech cluster, which MedTech Flanders is part of together with Flanders, Bio, and DSP? Is there a good evolution? Should a start-up become a member at an early stage to get quicker go to markets, or was Scilife support sufficient?
A: I think that's a very good question because I did approach the fact of grant funding for one. But I think another thing that's been very valuable in our go-to-market strategy is being part of these cluster organizations. So initially Flanders Bio, we're also a member of Midday Flanders now of the larger cluster, and that aids because they really help, they know the playing field of the industry. They help you by going to conferences, there are even some subsidies, that you can apply for and, that's mostly done with Flanders investment and trade. I think it's a very good way to better understand, the different markets by being part of these organizations.
I would encourage everyone to be part of those because it helps your go-to-market strategy and to gain commercial trajectory a lot quicker in the process.
Q: Any high-level tips on how to move from research to market faster?
A: I think talking to people, networking, really starting to understand who our customers are, how can we gain their trust, how can we get them to buy our product and, doing all of those outreach communications.
I think that's very helpful and, really needed, otherwise you just can continue working on the development forever. You will always see new improvements coming up, but ultimately you just want to move the product into the markets. So, talking to people early on, I think it's, really key to shorten your trajectory and move into that sales organization.
It's all about customer satisfaction and making sure we get high-quality products out there.
Q: Is it possible to get to CE Marking before being ISO 13485 Certified?
A: Yes, that's indeed also what Novosanis did. They obtained CE marking before being ISO 13485 certified
Q: How long does it take to obtain the CE marking from the time the TEchnical File is completed? Class A?
A: Class A: max 30 days.
Q: Who helped you to get to the right EU funding programs?
A: EEN = Enterprise Europe Network, the NCP (national contact point) and grant writers, next to own expertise with funding channels.
Q: How do you know which specific MD/IVD falls under which category? Is there a list available where this classification can be checked (classification under the Directives and new Regulations)? For example, the Covid antigen tests that are being placed on the market.
A: Yes, there are decision trees available. Under IVDR:
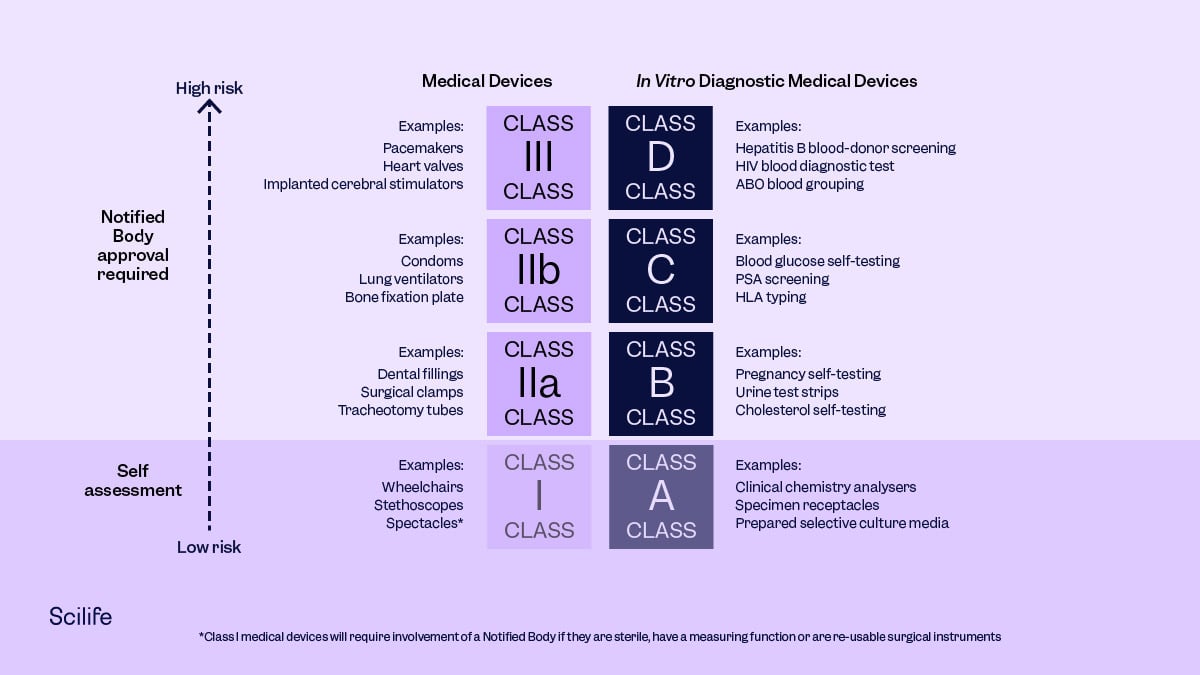
Q: Just to come back on the classification, what if it is not mentioned on the DoC, can this be found somewhere else? For example, the Covid antigen tests that are being distributed on the market now, what classification of IVD are they?
A: They’re still self-certification now; this will change under IVDR.
Q: Why is your QMS certified for 13485:2003, not 2016?
A: We have 2016 now, but when we obtained ISO it was 2003, so the slide reflected obtaining our certification for the first time.
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
PRODUCT
INDUSTRIES
RESOURCES
COMPANY
Contact Us
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2026 Scilife N.V. All rights reserved.